Abstract
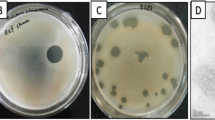
Over the last years, the global production and trade of kiwifruit has been severely impacted by Pseudomonas syringae pv. actinidiae (Psa), a phytopathogen that causes a disease in kiwifruit plants known as bacterial canker. The available treatments for this disease are still scarce, with the most common involving frequently spraying the orchards with disinfectants, copper-based bactericides and/or antibiotics. Moreover, these treatments should be avoided due to their high toxicity to the environment and promotion of bacterial resistance. Phage therapy may be an alternative approach to inactivate Psa. The present study investigated the potential application of the already commercially available bacteriophage (or phage) ϕ6 to control Psa infections. The inactivation of Psa was assessed in vitro, using liquid culture medium, and ex vivo, using artificially contaminated kiwifruit leaves with two biovar 3 (a highly aggressive pathogen) strains (Psa CRA-FRU 12.54 and Psa CRA-FRU 14.10). In the in vitro experiments, the phage ϕ6 was effective against both strains (maximum reduction of 2.2 and 1.9 CFU/mL for Psa CRA-FRU 12.54 and Psa CRA-FRU 14.10, respectively). In the ex vivo tests, the decrease was lower (maximum reduction 1.1 log and 1.8 CFU/mL for Psa CRA-FRU 12.54 and Psa CRA-FRU 14.10, respectively). The results of this study suggest that the commercially available phage ϕ6 can be an effective alternative to control Psa infections in kiwifruit orchards.






Similar content being viewed by others
References
Abuladze T, Li M, Menetrez MY, Dean T, Senecal A, Sulakvelidze A (2008) Bacteriophages reduce experimental contamination of hard surfaces, tomato, spinach, broccoli, and ground beef by Escherichia coli O157:H7. Appl Environ Microbiol 74:6230–6238
Adams MH (1959) Bacteriophages. Interscience Publishers, John Wiley and Sons Inc, New York
Almeida A, Cunha Â, Gomes NCM, Alves E, Costa L, Faustino MAF (2009) Phage therapy and photodynamic therapy : low environmental impact approaches to inactivate microorganisms in fish farming plants. Mar Drugs 7:268–313
Altimira F, Yanez C, Bravo G, Gonzalez M, Rojas LA, Seeger M (2012) Characterization of copper-resistant bacteria and bacterial communities from copper-polluted agricultural soils of Central Chile. BMC Microbiol 12:1–12
Bae YJ, Wu J, Lee HJ, Jo EJ, Murugaiyan S, Chung E, Lee SW (2012) Biocontrol potential of a lytic bacteriophage PE204 against bacterial wilt of tomato. J Microbiol Biotechnol 22:1613–1620
Balcão VM, Vieira MC, Malcata FX (1996) Adsorption of protein from several commercial lipase preparations onto a hollow-fiber membrane module. Biotechnol Prog 12:164–172
Balcão VM, Oliveira TA, Xavier Malcata F (1998) Stability of a commercial lipase from Mucor javanicus: kinetic modelling of pH and temperature dependencies. Biocatal Biotransfor 16:45–66
Balestra GM, Renzi M, Mazzaglia A (2010) First report of bacterial canker of Actinidia deliciosa caused by Pseudomonas syringae pv. actinidiae in Portugal. New Dis Rep 22:10
Balestra GM, Renzi M, Mazzaglia A (2011) First report of Pseudomonas syringae pv. actinidiae on kiwifruit plants in Spain. New Dis Rep 24:10
Balogh B, Jones JB, Momol MT, Olson SM, Obradovic A, King P, Jackson LE (2003) Improved efficacy of newly formulated bacteriophages for management of bacterial spot on tomato. Plant Dis 87:949–954
Baolin S, Chenghua W, Jing Z, Luxi L, Qiguo Z (2016) Geographical distributions of Pseudomonas syringae pv. actinidiae in China. Plant Prot 42:146–150
Bates DM, Watts DG (1988) Nonlinear regression analysis and its applications, 1st edn. Wiley Interscience. The University of Michigan, Volume 181 of Wiley Series in Probability and Statistics - Applied Probability and Statistics Section Series, 365 pages
Bender CL, Alarcon-Chaidez F, Gross DC (1999) Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol Mol Biol Rev 63:266–292
Callanan J, Stockdale SR, Shkoporov A, Draper LA, Ross RP, Hill C (2018) RNA phage biology in a metagenomic era. Viruses 10:1–17
Cameron A, Sarojini V (2014) Pseudomonas syringae pv. actinidiae: chemical control , resistance mechanisms and possible alternatives. Plant Pathol 63:1–11
Corrado L, González-Ballesteros N, Scortichini M, Rodríguez-Argüelles MC, Gallego PP, Barreal ME (2018) Comparison of the effectiveness of several commercial products and two new copper complexes to control Pseudomonas syringae pv. actinidiae. In: Acta Horticulturae. International Society for Horticultural Science (ISHS), Leuven, Belgium, pp 247–252
Costa P, Pereira C, Gomes A, Almeida A (2019) Efficiency of single phage suspensions and phage cocktail in the inactivation of Escherichia coli and Salmonella Typhimurium: an in vitro preliminary study. Microorganisms 7:94
Czajkowski R, Ozymko Z, Lojkowska E (2014) Isolation and characterization of novel soilborne lytic bacteriophages infecting Dickeya spp. biovar 3 (‘D. solani’). Plant Pathol 63:758–772
Duarte J, Pereira C, Moreirinha C, Salvio R, Lopes A, Wang D, Almeida A (2018) New insights on phage efficacy to control Aeromonas salmonicida in aquaculture systems: an in vitro preliminary study. Aquaculture 495:970–982
Eman OH, Afaf Z (2014) Biocontrol of halo blight of bean caused by Pseudomonas phaseolicola. Int J Virol 10:235–242
Everett KR, Cohen D, Pushparajah IPS, Vergara MJ, Curtis CL, Larsen NJ, Jia Y (2012) Heat treatments to kill Pseudomonas syringae pv. actinidiae on contaminated pollen. New Zeal Plant Prot 65:8–18
Ferrante P, Scortichini M (2010) Molecular and phenotypic features of Pseudomonas syringae pv. actinidiae isolated during recent epidemics of bacterial canker on yellow kiwifruit (Actinidia chinensis) in Central Italy. Plant Pathol 59:954–962
Firrao G, Torelli E, Polano C, Ferrante P, Ferrini F, Martini M, Marcelletti S, Scortichini M, Ermacora P (2018) Genomic structural variations affecting virulence during clonal expansion of Pseudomonas syringae pv. actinidiae biovar 3 in Europe. Front Microbiol 9:1–13
Flaherty JE, Jones J, Harbaugh BK, Somodi GC, Jackson LE (2000) Control of bacterial spot on tomato in the greenhouse and field with H-mutant bacteriophages. Hortscience 35:882–884
Fong K, LaBossiere B, Switt A, Delaquis P, Goodridge L, Levesque R, Danyluk M, Wang S (2017) Characterization of four novel bacteriophages isolated from British Columbia for control of non-typhoidal Salmonella in vitro and on sprouting alfalfa seeds. Front Microbiol 8:2193
Frampton RA, Pitman AR, Fineran PC (2012) Advances in bacteriophage-mediated control of plant pathogens. Int J Microbiol 2012:1–11
Frampton R, Taylor C, Holguin Moreno A, Visnovsky S, Petty N, Pitman A, Fineran P (2014) Identification of bacteriophages for biocontrol of the kiwifruit canker phytopathogen Pseudomonas syringae pv. actinidiae. Appl Environ Microbiol 80:2216–2228
Fujikawa T, Sawada H (2019) Genome analysis of Pseudomonas which produces the phytotoxins, phaseolotoxin and coronatine. Sci Rep 9:1–11
Fujiwara A, Fujisawa M, Hamasaki R, Kawasaki T, Fujie M, Yamada T (2011) Biocontrol of Ralstonia solanacearum by treatment with lytic bacteriophages. Appl Environ Microbiol 77:4155–4162
García R, Latz S, Romero J, Higuera G, García K, Bastías R (2019) Bacteriophage production models: an overview. Front Microbiol 10:1–7
Gill JJ, Hyman P (2010) Phage choice, isolation, and preparation for phage therapy. Curr Pharm Biotechnol 11:2–14
Guroo I, Wani S, Wani S, Ahmad M, Mir S, Masoodi F (2017) A review of production and processing of kiwifruit. J Food Process Technol 8:1–6
Hwang MSH, Morgan RL, Sarkar SF, Wang PW, Guttman DS (2005) Phylogenetic characterization of virulence and resistance phenotypes of Pseudomonas syringae. Appl Environ Microbiol 71:5182–5191
Jesus V, Martins D, Branco T, Valerio N, Neves MGPMS, Faustino MAF, Reis L, Barreal E, Gallego PP, Almeida A (2018) An insight into the photodynamic approach versus copper formulations in the control of Pseudomonas syringae pv. actinidiae in kiwi plants. Photochem Photobiol Sci 17:180–191
Kalpage MD, De Costa DM (2014) Isolation of bacteriophages and determination of their efficiency in controlling Ralstonia solanacearum causing bacterial wilt of tomato. Trop Agric Res 26:140–151
Kim M-H, Park S-W, Kim Y-K (2011) Bacteriophages of Pseudomonas tolaasii for the biological control of brown blotch disease. J Korean Soc Appl Biol Chem 54:99–104
Koh YJ, Park S, Lee D (1996) Characteristics of bacterial canker of kiwifruit occurring in Korea and its control by trunk injection. Korean J Plant Pathol 12:324–330
Koh YJ, Kim GH, Jung JS, Lee YS, Hur JS (2010) Outbreak of bacterial canker on Hort16A (Actinidia chinensis Planchon) caused by Pseudomonas syringae pv. actinidiae in Korea. New Zeal J Crop Hortic Sci 38:275–282
Lindberg H, McKean K, Wang I (2014) Phage fitness may help predict phage therapy efficacy. Bacteriophage 4:e964081
Liu Y, Zhu T, Fan F, Shao B (2013) Occurrence and pathogen identification of kiwifruit bacterial canker in Sichuan. Hubei Agric Sci 52:4937–4941
Maleki S, Maleki-Zanjani B, Gallego PP (2018) Kiwifruit status in Iran: management and production. Acta Hortic 1218:39–44
Mäntynen S, Sundberg L-R, Poranen MM (2018) Recognition of six additional cystoviruses: Pseudomonas virus phi6 is no longer the sole species of the family Cystoviridae. Arch Virol 163:1117–1124
Marcelletti S, Ferrante P, Petriccione M, Firrao G, Scortichini M (2011) Pseudomonas syringae pv. actinidiae draft genomes comparison reveal strain-specific features involved in adaptation and virulence to Actinidia species. PLoS One 6:e27297
Martins D, Mesquita MQ, Neves MGPMS, Faustino MAF, Reis L, Figueira E, Almeida A (2018) Photoinactivation of Pseudomonas syringae pv. actinidiae in kiwifruit plants by cationic porphyrins. Planta 248:409–421
Mateus L, Costa L, Silva YJ, Pereira C, Cunha A, Almeida A (2014) Efficiency of phage cocktails in the inactivation of Vibrio in aquaculture. Aquaculture 424–425:167–173
McCann HC, Rikkerink EHA, Bertels F, Fiers M, Lu A, Rees-George J, Andersen MT, Gleave AP, Haubold B, Wohlers MW, Guttman DS, Wang PW, Straub C, Vanneste J, Rainey PB, Templeton MD (2013) Genomic analysis of the kiwifruit pathogen Pseudomonas syringae pv. actinidiae provides insight into the origins of an emergent plant disease. PLoS Pathog 9:e1003503
McCann HC, Li L, Liu Y, Li D, Pan H, Zhong C, Rikkerink EHA, Templeton MD, Straub C, Colombi E, Rainey PB, Huang H (2017) Origin and evolution of the kiwifruit canker pandemic. Genome Biol Evol 9:932–944
Monk AB, Rees CD, Barrow P, Hagens S, Harper DR (2010) Bacteriophage applications: where are we now? Lett Appl Microbiol 51:363–369
Moye ZD, Woolston J, Sulakvelidze A (2018) Bacteriophage applications for food production and processing. Viruses 10:1–22
Nguyen HTD, Yoon S, Kim M-H, Kim Y-K, Yoon M-Y, Cho Y-H, Lim Y, Shin SH, Kim D-E (2012) Characterization of bacteriophage ϕPto-bp6g, a novel phage that lyses Pseudomonas tolaasii causing brown blotch disease in mushrooms. J Microbiol Methods 91:514–519
Oliveira H, São-José C, Azeredo J (2018) Phage-derived peptidoglycan degrading enzymes: challenges and future prospects for in vivo therapy. Viruses 10:292
Park J, Lim J-A, Yu J-G, Oh C-S (2018) Genomic features and lytic activity of the bacteriophage PPPL-1 effective against Pseudomonas syringae pv. actinidiae, a cause of bacterial canker in kiwifruit. J Microbiol Biotechnol 28:1542–1546
Pereira C, Silva YJ, Santos AL, Cunha Â, Gomes NCM, Almeida A (2011) Bacteriophages with potential for inactivation of fish pathogenic bacteria: Survival , host specificity and effect on bacterial community structure. Mar Drugs 9:2236–2255
Pereira C, Moreirinha C, Rocha RJM, Calado R, Romalde JL, Nunes ML, Almeida A (2016a) Application of bacteriophages during depuration reduces the load of Salmonella Typhimurium in cockles. Food Res Int 90:73–84
Pereira C, Moreirinha C, Lewicka M, Almeida P, Clemente C, Cunha Â, Delgadillo I, Romalde JL, Nunes ML, Almeida A (2016b) Bacteriophages with potential to inactivate Salmonella Typhimurium: use of single phage suspensions and phage cocktails. Virus Res 220:179–192
Pereira C, Moreirinha C, Teles L, Rocha RJM, Calado R, Romalde JL, Nunes ML, Almeida A (2017a) Application of phage therapy during bivalve depuration improves Escherichia coli decontamination. Food Microbiol 61:102–112
Pereira C, Moreirinha C, Lewicka M, Almeida P, Clemente C, Romalde JL, Nunes ML, Almeida A (2017b) Characterization and in vitro evaluation of new bacteriophages for the biocontrol of Escherichia coli. Virus Res 227:171–182
Pinheiro LAM, Pereira C, Frazão C, Balcão VM, Almeida A (2019) Efficiency of phage ϕ6 for biocontrol of Pseudomonas syringae pv. syringae: an in vitro preliminary study. Microorganisms 7:286
Pires D, Oliveira H, Melo L, Sillankorva S, Azeredo J (2016) Bacteriophage-encoded depolymerases: their diversity and biotechnological applications. Appl Microbiol Biotechnol 100:2141–2151
Poulter RTM, Ho J, Handley T, Taiaroa G, Butler MI (2018) Comparison between complete genomes of an isolate of Pseudomonas syringae pv. actinidiae from Japan and a New Zealand isolate of the pandemic lineage. Sci Rep 8:1–13
Rios A, Moutinho C, Pinto F, Del Fiol F, Jozala A, Chaud M, Vila M, Teixeira J, Balcão V (2016) Alternatives to overcoming bacterial resistances: state-of-the-art. Microbiol Res 191:51–80
Santos SB, Carvalho C, Azeredo J, Ferreira EC (2014) Population dynamics of a Salmonella lytic phage and its host: implications of the host bacterial growth rate in modelling. PLoS One 9:e102507
Scortichini M (1994) Occurrence of Pseudomonas syringae pv. actinidiae on kiwifruit in Italy. Plant Pathol 43:1035–1038
Shao Y, Wang I-N (2008) Bacteriophage adsorption rate and optimal lysis time. Genetics 180:471–482
Silva Y, Costa L, Pereira C, Mateus C, Cunha Â, Calado R, Gomes N, Pardo M, Hernandez I, Almeida A (2014) Phage therapy as an approach to prevent Vibrio anguillarum infections in fish larvae production. PLoS One 9:e114197
Silva Y, Moreirinha C, Pereira C, Costa L, Rocha RJM, Cunha Â, Gomes NCM, Calado R, Almeida A (2016) Biological control of Aeromonas salmonicida infection in juvenile Senegalese sole (Solea senegalensis) with phage AS-A. Aquaculture 450:225–233
Soffer N, Woolston J, Li M, Das C, Sulakvelidze A (2017) Bacteriophage preparation lytic for Shigella significantly reduces Shigella sonnei contamination in various foods. PLoS One 12:e0175256
Stuer-Lauridsen B, Janzen T, Schnabl J, Johansen E (2003) Identification of the host determinant of two prolate-headed phages infecting Lactococcus lactis. Virology 309:10–17
Takikawa Y, Serizawa S, Ichikawa T, Tsuyumu S, Goto M (1989) Pseudomonas syringae pv. actinidae pv. nov.: the causal bacterium of canker of kiwifruit in Japan. Jpn J Phytopathol 55:437–444
Vanneste J (2012) Pseudomonas syringae pv. actinidiae (Psa): a threat to the New Zealand and global kiwifruit industry. New Zeal J Crop Hortic Sci 40:265–267
Vanneste J, Yu J, Cornish D, Tanner D, Windner R, Chapman J, Taylor R, Mackay J, Dowlut S (2013) Identification, virulence, and distribution of two biovars of Pseudomonas syringae pv. actinidiae in New Zealand. Plant Dis 97:708–719
Vidaver AK, Koski RK, Van Etten JL (1973) Bacteriophage phi6: a lipid-containing virus of Pseudomonas phaseolicola. J Virol 11:799–805
Vieira A, Silva YJ, Cunha A, Gomes NCM, Ackermann H-W, Almeida A (2012) Phage therapy to control multidrug-resistant Pseudomonas aeruginosa skin infections: in vitro and ex vivo experiments. Eur J Clin Microbiol Infect Dis 31:3241–3249
Wei H, Cheng RH, Berriman J, Rice WJ, Stokes DL, Katz A, Morgan DG, Gottlieb P (2009) Three-dimensional structure of the enveloped bacteriophage Φ12: an incomplete T = 13 lattice is superposed on an enclosed T = 1 shell. PLoS One 4:e6850
Wickner RB (1993) Double-stranded RNA virus replication and packaging. J Biol Chem 268:3797–3800
Wilstermann A, Schrader G, Kehlenbeck H, Robinet C (2017) Potential spread of kiwifruit bacterial canker (Pseudomonas syringae pv. actinidiae) in Europe. EPPO Bull 47:255–262
Yang Y, Lu S, Shen W, Zhao X, Shen M, Tan Y, Li G, Li M, Wang J, Hu F, Le S (2016) Characterization of the first double-stranded RNA bacteriophage infecting Pseudomonas aeruginosa. Sci Rep 6:38795
Yu J, Lim J, Song Y, Heu S, Kim G, Koh Y, Oh C (2016) Isolation and characterization of bacteriophages against Pseudomonas syringae pv. actinidiae causing bacterial canker disease in kiwifruit. J Microbiol Biotechnol 26:385–393
Acknowledgements
Thanks are also due to the Department of Biology and University of Aveiro where this research work was carried out.
Funding
FCT/MCTES provided financial support to CESAM (UID/AMB/50017/2019), through national funds. Carla Pereira was supported by a Junior Research contract (CEEC Individual/03974/2017) financed by the Portuguese Foundation for Science and Technology (FCT). Project funding by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo, Brazil) (BPE fellowship Ref. No. 2018/05522-9, Project PsaPhageKill, granted to Victor M. Balcão) is hereby gratefully acknowledged. This work also received support from CNPq, National Council for Scientific and Technological Development Brazil, in the form of Research Productivity (PQ) fellowships granted to Victor M. Balcão (Refs. No. 306113/2014-7 and 308208/2017-0).
Author information
Authors and Affiliations
Contributions
Larindja A. M. Pinheiro, Carla Pereira and Victor M. Balcão performed the experiments. Larindja A. M. Pinheiro, Victor M. Balcão and Carla Pereira prepared the paper. M. Esther Barreal, Pedro Pablo Gallego revised the paper and contributed with Psa strains. Adelaide Almeida supervised the work, prepared and revised the paper and contributed with reagents and analysis tools.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pinheiro, L.A.M., Pereira, C., Barreal, M.E. et al. Use of phage ϕ6 to inactivate Pseudomonas syringae pv. actinidiae in kiwifruit plants: in vitro and ex vivo experiments. Appl Microbiol Biotechnol 104, 1319–1330 (2020). https://doi.org/10.1007/s00253-019-10301-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10301-7