Abstract
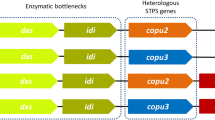
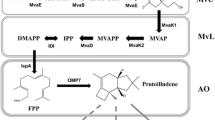
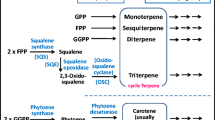
Ophiobolins (ophs) are characteristic 5-8-5 tricyclic sesterterpenes with potential pharmaceutical activities. Ophiobolin synthase is a bifunctional terpene synthase (BTS) that catalyzes both chain elongation and cyclization. In Aspergillus ustus 094102, ophiobolin accumulation was involved with not only ophiobolin synthase C25 (Au8003) but also other four gene clusters containing C15 (Au6298), C20 (Au13192 and Au11565), and C30 (Au3446) terpene synthases. In this report, overexpression of codon-optimized gene Au8003 resulted in a detectable production of oph F in E. coli. In subsequent modulation of culture conditions, pentose arabinose allowed a more than 10-fold improvement of production than that of glycerol. To achieve a higher titer, the whole mevalonate pathway and an additional copy of isopentenyl diphosphate isomerase gene were assembled, leading to approximately 24-fold and 60-fold yield increases, respectively. The above four terpene synthase genes related to ophiobolin production in strain 094102 were individually or combinatorially overexpressed with Au8003 to mimic the original fungal biosynthesis. The biosynthesis of oph scaffold was increased by short-chain terpene synthases (C15 and C20), among which the C15 synthase gene contributed the highest yield of 82.76 mg/L at 96 h; the multi-gene combinatorial results suggested that cyclization might be a rate-limiting step. Further protein engineering including fusion tags and phylogenetically based mutations on the rate-limiting cyclization part of Au8003 enabled a further yield improvement (> 150 mg/L at 96 h) in shake flasks. These multiple approaches for sesterterpene skeleton production using engineered E. coli may be applicable for cost-effective, high-yield productions of ophiobolins and other compounds synthesized by BTSs.







Similar content being viewed by others
References
Abdallah II, Ronald VM, Klumpenaar E, Quax WJ (2018) Catalysis of amorpha-4,11-diene synthase unraveled and improved by mutability landscape guided engineering. Sci Rep 8(9961):9961. https://doi.org/10.1038/s41598-018-28177-4
Arens J, Engels B, Klopries S, Jennewein S, Ottmann C, Schulz F (2013) Exploration of biosynthetic access to the shared precursor of the fusicoccane diterpenoid family. Chem Commun 49:4337–4339. https://doi.org/10.1039/c2cc37154e
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201. https://doi.org/10.1093/bioinformatics/bti770
Bian G, Deng Z, Liu T (2017) Strategies for terpenoid overproduction and new terpenoid discovery. Curr Opin Biotechnol 48:234–241. https://doi.org/10.1016/j.copbio.2017.07.002
Brill ZG, Grover HK, Maimone TJ (2016) Enantioselective synthesis of an ophiobolin sesterterpene via a programmed radical cascade. Science 352:1078–1082. https://doi.org/10.1126/science.aaf6742
Bury M, Girault A, Megalizzi V, Spiegl-Kreinecker S, Mathieu V, Berger W, Evidente A, Kornienko A, Gailly P, Vandier C, Kiss R (2013) Ophiobolin A induces paraptosis-like cell death in human glioblastoma cells by decreasing BKCa channel activity. Cell Death Dis 4:e561. https://doi.org/10.1038/cddis.2013.85
Chai H, Yin R, Liu Y, Meng H, Zhou X, Zhou G, Bi X, Yang X, Zhu T, Zhu W, Deng Z, Hong K (2016) Sesterterpene ophiobolin biosynthesis involving multiple gene clusters in Aspergillus ustus. Sci Rep 6:27181. https://doi.org/10.1038/srep27181
Chen W, Ye L, Guo F, Lv Y, Yu H (2015a) Enhanced activity of an alkaline phytase from Bacillus subtilis 168 in acidic and neutral environments by directed evolution. Biotech Eng J 98:137–143. https://doi.org/10.1016/j.bej.2015.02.021
Chen X, Shi J, Chen R, Wen Y, Shi Y, Zhu Z, Guo S, Li L (2015b) Molecular chaperones (TrxA, SUMO, Intein, and GST) mediating expression, purification, and antimicrobial activity assays of plectasin in Escherichia coli. Biotechnol Appl Biochem 62:606–614. https://doi.org/10.1002/bab.1303
Chen M, Chou WK, Toyomasu T, Cane DE, Christianson DW (2016) Structure and function of fusicoccadiene synthase, a hexameric bifunctional diterpene synthase. ACS Chem Biol 11:889–899. https://doi.org/10.1021/acschembio.5b00960
Chiba R, Minami A, Gomi K, Oikawa H (2013) Identification of ophiobolin F synthase by a genome mining approach: a sesterterpene synthase from Aspergillus clavatus. Org Lett 15:594–597. https://doi.org/10.1021/ol303408a
Christianson DW (2017) Structural and chemical biology of terpenoid cyclases. Chem Rev 117:11570–11648. https://doi.org/10.1021/acs.chemrev.7b00287
Crooks GE, Hon G, Chandonia JM, Brenner SE (2004) WebLogo: A sequence logo generator. Genome Res 14:1188–1190. https://doi.org/10.1101/gr.849004
George KW, Alonso-Gutierrez J, Keasling JD, Lee TS (2015) Isoprenoid drugs, biofuels, and chemicals-artemisinin, farnesene, and beyond. In: Schrader J, Bohlmann J (eds) Biotechnology of Isoprenoids. Adv. Biochem. Eng./Biotechnol, vol 148. Springer, Cham, pp 355–389. https://doi.org/10.1007/10_2014_288
George KW, Thompson MG, Kim J, Baidoo EE, Wang G, Benites VT, Petzold CJ, Chan LJG, Yilmaz S, Turhanen P (2018) Integrated analysis of isopentenyl pyrophosphate (IPP) toxicity in isoprenoid-producing Escherichia coli. Metab Eng 47:60–72. https://doi.org/10.1016/j.ymben.2018.03.004
Hong K, Yang X (2013) High-yield ophiobolin compound strain Aspergillus ustus TKYX429 and application thereof. China Patent: ZL201310186483.4. 2013-8-7
Leonard E, Ajikumar PK, Thayer K, Xiao WH, Mo JD, Tidor B, Stephanopoulos G, Prather KLJ (2010) Combining metabolic and protein engineering of a terpenoid biosynthetic pathway for overproduction and selectivity control. Proc Natl Acad Sci U S A 107:13654–13659. https://doi.org/10.1073/pnas.1006138107
Li ZJ, Hong PH, Da YY, Li LK, Stephanopoulos G (2018) Metabolic engineering of Escherichia coli for the production of L-malate from xylose. Metab Eng 48:25–32. https://doi.org/10.1016/j.ymben.2018.05.010
Liu C, Bi H, Bai Z, Fan L, Tan T (2019) Engineering and manipulation of a mevalonate pathway in Escherichia coli for isoprene production. Appl Microbiol Biotechnol 103:239–250. https://doi.org/10.1007/s00253-018-9472-9
Mandell DJ, Coutsias EA, Kortemme T (2009) Sub-angstrom accuracy in protein loop reconstruction by robotics-inspired conformational sampling. Nat Methods 6:551–552. https://doi.org/10.1038/nmeth0809-551
Martin VJ, Pitera DJ, Withers ST, Newman JD, Keasling JD (2003) Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat Biotechnol 21:796–802. https://doi.org/10.1038/nbt833
Masi M, Dasari R, Evidente A, Mathieu V, Kornienko A (2019) Chemistry and biology of ophiobolin A and its congeners. Bioorg Med Chem Lett 29:859–869. https://doi.org/10.1016/j.bmcl.2019.02007
Matsuda Y, Mitsuhashi T, Lee S, Hoshino M, Mori T, Okada M, Zhang H, Hayashi F, Fujita M, Abe I (2016) Astellifadiene: Structure determination by NMR spectroscopy and crystalline sponge method, and elucidation of its biosynthesis. Angew Chem Int Ed 55:5785–5788. https://doi.org/10.1002/anie.201601448
Narita K, Sato H, Minami A, Kudo K, Gao L, Liu C, Ozaki T, Kodama M, Lei X, Taniguchi T, Monde K, Yamazaki M, Uchiyama M, Oikawa H (2017) Focused genome mining of structurally related sesterterpenes: enzymatic formation of enantiomeric and diastereomeric products. Org Lett 19:6696–6699. https://doi.org/10.1021/acs.orglett.7b03418
Nogué VS, Karhumaa K (2015) Xylose fermentation as a challenge for commercialization of lignocellulosic fuels and chemicals. Biotechnol Lett 37:761–772. https://doi.org/10.1007/s10529-014-1756-2
Okada M, Matsuda Y, Mitsuhashi T, Hoshino S, Mori T, Nakagawa K, Quan Z, Qin B, Zhang H, Hayashi F (2016) Genome-based discovery of an unprecedented cyclization mode in fungal sesterterpenoid biosynthesis. J Am Chem Soc 138:10011–10018. https://doi.org/10.1021/jacs.6b05799
Paddon CJ, Keasling JD (2014) Semi-synthetic artemisinin: a model for the use of synthetic biology in pharmaceutical development. Nat Rev Microbiol 12:355–367. https://doi.org/10.1038/nrmicro3240
Paramasivan K, Mutturi S (2017) Regeneration of NADPH coupled with HMG-CoA reductase activity increases squalene synthesis in Saccharomyces cerevisiae. J Agric Food Chem 65:8162–8170. https://doi.org/10.1021/acs.jafc.7b02945
Pereira B, Li ZJ, De Mey M, Lim CG, Zhang H, Hoeltgen C, Stephanopoulos G (2016) Efficient utilization of pentoses for bioproduction of the renewable two-carbon compounds ethylene glycol and glycolate. Metab Eng 34:80–87. https://doi.org/10.1016/j.ymben.2015.12.004
Qin B, Matsuda Y, Mori T, Okada M, Quan Z, Mitsuhashi T, Wakimoto T, Abe I (2016) An unusual chimeric diterpene synthase from Emericella variecolor and its functional conversion into a sesterterpene synthase by domain swapping. Angew Chem Int Ed 55:1658–1661. https://doi.org/10.1002/anie.201509263
Rowley M, Tsukamoto M, Kishi Y (1989) Total synthesis of (+)-ophiobolin C. J Am Chem Soc 111:2735–2737. https://doi.org/10.1021/ja00189a069
Sato H, Narita K, Minami A, Yamazaki M, Wang C, Suemune H, Nagano S, Tomita T, Oikawa H, Uchiyama M (2018) Theoretical study of sesterfisherol biosynthesis: computational prediction of key amino acid residue in terpene synthase. Sci Rep 8:2473. https://doi.org/10.1038/s41598-018-20916-x
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) General atomic and molecular electronic-structure system. J Comput Chem 14:1347–1363. https://doi.org/10.1002/jcc.540141112
Stein A, Kortemme T (2013) Improvements to robotics-inspired conformational sampling in rosetta. PLoS One 8:e63090. https://doi.org/10.1371/journal.pone.0063090
Steinmetz EJ, Auldridge ME (2017) Screening fusion tags for improved recombinant protein expression in E. coli with the Expresso® solubility and expression screening system. Curr Protoc Protein Sci 90:5–27. https://doi.org/10.1002/cpps.39
Sun W, Lv C, Zhu T, Yang X, Wei S, Sun J, Hong K, Zhu W, Huang C (2013) Ophiobolin-O reverses adriamycin resistance via cell cycle arrest and apoptosis sensitization in adriamycin-resistant human breast carcinoma (MCF-7/ADR) cells. Mar Drugs 11:4570–4584. https://doi.org/10.3390/md11114570
Tian W, Deng Z, Hong K (2017) The biological activities of sesterterpenoid-type ophiobolins. Mar Drugs 15:229. https://doi.org/10.3390/md15070229
Toyomasu T, Tsukahara M, Kaneko A, Niida R, Mitsuhashi W, Dairi T, Kato N, Sassa T (2007) Fusicoccins are biosynthesized by an unusual chimera diterpene synthase in fungi. Proc Natl Acad Sci U S A 104:3084–3088. https://doi.org/10.1073/pnas.0608426104
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334
Tsuna K, Noguchi N, Nakada M (2011) Convergent total synthesis of (+)-ophiobolin A. Angew Chem Int Ed 50:9624–9627. https://doi.org/10.1002/anie.201104447
Wada K, Toya Y, Banno S, Yoshikawa K, Matsuda F, Shimizu H (2017) 13C-metabolic flux analysis for mevalonate-producing strain of Escherichia coli. J Biosci Bioeng 123:177–182. https://doi.org/10.1016/j.jbiosc.2016.08.001
Wang M, Chen B, Fang Y, Tan T (2017) Cofactor engineering for more efficient production of chemicals and biofuels. Biotechnol Adv 35:1032–1039. https://doi.org/10.1016/j.biotechadv.2017.09.008
Yang H, Liu L, Xu F (2016) The promises and challenges of fusion constructs in protein biochemistry and enzymology. Appl Microbiol Biotechnol 100:8273–8281. https://doi.org/10.1007/s00253-016-7795-y
Zhu F, Zhong X, Hu M, Lu L, Deng Z, Liu T (2014) In vitro reconstitution of mevalonate pathway and targeted engineering of farnesene overproduction in Escherichia coli. Biotechnol Bioeng 111:1396–1405. https://doi.org/10.1002/bit.25198
Zhu T, Lu Z, Fan J, Wang L, Zhu G, Wang Y, Li X, Hong K, Piyachaturawat P, Chairoungdua A, Zhu W (2018) Ophiobolins from the mangrove fungus Aspergillus ustus. J Nat Prod 81:2–9. https://doi.org/10.1021/acs.jnatprod.7b00335
Acknowledgments
The authors are grateful to professor Tiangang Liu in Wuhan University for pMH1 and pFZ81 plasmids. We acknowledge Mr. Guofu Qiu in Wuhan University for NMR data collection. We are grateful to the assistance of Miss Yanyu Tao, Shiyu Zhu, and Mr. Jiangfeng Lu for fermentation experiments.
Funding
This work was financially supported by grants from the National Key Research and Development Program of China (No. 2018YFC0311001) and National Natural Science Foundation of China (No. 81673331).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Wuhan University has filed a patent application based on this work.
Ethical statement
This article does not contain any studies involving human participants or experimental animals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1751 kb)
Rights and permissions
About this article
Cite this article
Yuan, W., Lv, S., Chen, L. et al. Production of sesterterpene ophiobolin by a bifunctional terpene synthase in Escherichia coli. Appl Microbiol Biotechnol 103, 8785–8797 (2019). https://doi.org/10.1007/s00253-019-10103-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10103-x