Abstract
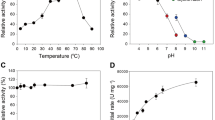
Biomass hydrolysis constitutes a bottleneck for the biotransformation of lignocellulosic residues into bioethanol and high-value products. The efficient deconstruction of polysaccharides to fermentable sugars requires multiple enzymes acting concertedly. GH43 β-xylosidases are among the most interesting enzymes involved in hemicellulose deconstruction into xylose. In this work, the structural and functional properties of β-xylosidase EcXyl43 from Enterobacter sp. were thoroughly characterized. Molecular modeling suggested a 3D structure formed by a conserved N-terminal catalytic domain linked to an ancillary C-terminal domain. Both domains resulted essential for enzymatic activity, and the role of critical residues, from the catalytic and the ancillary modules, was confirmed by mutagenesis. EcXyl43 presented β-xylosidase activity towards natural and artificial substrates while arabinofuranosidase activity was only detected on nitrophenyl α-L-arabinofuranoside (pNPA). It hydrolyzed xylobiose and purified xylooligosaccharides (XOS), up to degree of polymerization 6, with higher activity towards longer XOS. Low levels of activity on commercial xylan were also observed, mainly on the soluble fraction. The addition of EcXyl43 to GH10 and GH11 endoxylanases increased the release of xylose from xylan and pre-treated wheat straw. Additionally, EcXyl43 exhibited high efficiency and thermal stability under its optimal conditions (40 °C, pH 6.5), with a half-life of 58 h. Therefore, this enzyme could be a suitable additive for hemicellulases in long-term hydrolysis reactions. Because of its moderate inhibition by monomeric sugars but its high inhibition by ethanol, EcXyl43 could be particularly more useful in separate hydrolysis and fermentation (SHF) than in simultaneous saccharification and co-fermentation (SSCF) or consolidated bioprocessing (CBP).






Similar content being viewed by others
References
Brunzelle JS, Jordan DB, McCaslin DR, Olczak A, Wawrzak Z (2008) Structure of the two-subsite β-D-xylosidase from Selenomonas ruminantium in complex with 1,3 bis[tris(hydroxymethyl)methylamino]propane. Arch Biochem Bioph 474:157–166
Brüx C, Ben-David A, Shallom-Shezifi D, Leon M, Niefind K, Shoham G, Shoham Y, Schomburg D (2006) The structure of an inverting GH43 β-xylosidase from Geobacillus stearothermophilus with its substrate reveals the role of the three catalytic residues. J Mol Biol 359(1):97–109
Campos E, Negro Alvarez M, di Lorenzo G, Gonzalez S, Rorig M, Talia P, Grasso D, Sáez F, Manzanares Secades P, Ballesteros Perdices M, Cataldi A (2014) Purification and characterization of a GH43 β-xylosidase from Enterobacter sp. identified and cloned from forest soil bacteria. Microbiol Res 169(2–3):213–220
Diogo JA, Hoffmam ZB, Zanphorlin LM, Cota J, Machado CB, Wolf LD, Squina F, Dámasio A, Murakami M, Ruller R (2014) Development of a chimeric hemicellulase to enhance the xylose production and thermotolerance. Enzym Microb Technol 69:31–37
Dodd D, Cann IK (2009) Enzymatic deconstruction of xylan for biofuel production. GCB Bioenergy 1(1):2–17
Falck P, Linares-Pastén JA, Adlercreutz P, Karlsson EN (2016) Characterization of a family 43 β-xylosidase from the xylooligosaccharide utilizing putative probiotic Weissella sp. strain 92. Glycobiology 26(2):193–202
Fan Z, Yuan L, Jordan DB, Wagschal K, Heng C, Braker JD (2010) Engineering lower inhibitor affinities in β-D-xylosidase. Appl Microbiol Biotechnol 86(4):1099–1113
Ghio S, Ontañon OM, Piccinni FE, Marrero R, Talia P, Grasso DH, Campos E (2018) Paenibacillus sp. A59 GH10 and GH11 extracellular Endoxylanases: application in biomass bioconversion. BioEnergy Res 11:174–190
Gírio FM, Fonseca C, Carvalheiro F, Duarte LC, Marques S, Bogel-Łukasik R (2010) Hemicelluloses for fuel ethanol: a review. Bioresour Technol 101(13):4775–4800
Jordan DB, Li XL (2007) Variation in relative substrate specificity of bifunctional β-d-xylosidase/α-l-arabinofuranosidase by single-site mutations: roles of substrate distortion and recognition. Biochim Biophys Acta 1774(9):1192–1198
Jordan DB, Wagschal K, Grigorescu AA, Braker JD (2013) Highly active β-xylosidases of glycoside hydrolase family 43 operating on natural and artificial substrates. Appl Microbiol Biotechnol 97(10):4415–4428
Jørgensen H, Pinelo M (2017) Enzyme recycling in lignocellulosic biorefineries. Biofuels Bioprod Biorefin 11(1):150–167
Lagaert S, Pollet A, Delcour JA, Lavigne R, Courtin CM, Volckaert G (2011) Characterization of two β-xylosidases from Bifidobacterium adolescentis and their contribution to the hydrolysis of prebiotic xylooligosaccharides. Appl Microbiol Biotechnol 92(6):1179–1185
Lagaert S, Pollet A, Courtin CM, Volckaert G (2014) β-Xylosidases and α-L-arabinofuranosidases: accessory enzymes for arabinoxylan degradation. Biotechnol Adv 32(2):316–332
Lee CC, Braker JD, Grigorescu AA, Wagschal K, Jordan DB (2013) Divalent metal activation of a GH43 β-xylosidase. Enzym Microb Technol 52(2):84–90
Miller GL, Blum R, Glennon WE, Burton AL (1960) Measurement of carboxymethylcellulase activity. Anal Biochem 1(2):127–132
Morais S, Salama-Alber O, Barak Y, Hadar Y, Wilson DB, Lamed R, Bayer EA (2012) Functional association of catalytic and ancillary modules dictates enzymatic activity in glycoside hydrolase family 43 β-xylosidase. J Biol Chem 287(12):9213–9221
Nurizzo D, Nagy T, Gilbert HJ, Davies GJ (2002) The structural basis for catalysis and specificity of the Pseudomonas cellulosa alpha-glucuronidase GlcA67A. Structure 10(4):547–556
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF chimera-a visualization system for exploratory research and analysis. J Comput Chem 25(13):1605–1612
Proctor MR, Taylor EJ Nurizzo D, Turkenburg JP, Lloyd RM, Vardakou M, Davies GJ, Gilbert HJ (2005) Tailored catalysts for plant cell-wall degradation: redesigning the exo/endo preference of Cellvibrio japonicus arabinanase 43A. Proc Natl Acad Sci U S A 102(8):2697–2702
Roy A, Kucukural A, Zhang Y (2010) I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc 5(4):725–738
Saha BC (2003) Purification and properties of an extracellular β-xylosidase from a newly isolated Fusarium proliferatum. Bioresour Technol 90(1):33–38
Selig MJ, Knoshaug EP, Adney WS, Himmel ME, Decker SR (2008) Synergistic enhancement of cellobiohydrolase performance on pretreated corn Stover by addition of xylanase and esterase activities. Bioresour Technol 99(11):4997–5005
Shallom D, Leon M, Bravman T, Ben-David A, Zaide G, Belakhov V, Shoham G, Schomburg D, Baasov T, Shoham Y (2005) Biochemical characterization and identification of the catalytic residues of a family 43 β-D-xylosidase from Geobacillus stearothermophilus T-6. Biochemistry 44(1):387–397
Singh SK, Heng C, Braker JD, Chan VJ, Lee CC, Jordan DB, Yuan L, Wagschal K (2014) Directed evolution of GH43 β-xylosidase XylBH43 thermal stability and L186 saturation mutagenesis. J Ind Microbiol Biotechnol 41(3):489–498
Smaali I, Rémond C, O’Donohue MJ (2006) Expression in Escherichia coli and characterization of β-xylosidases GH39 and GH-43 from Bacillus halodurans C-125. Appl Microbiol Biotechnol 73(3):582–590
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729
Umemoto Y, Onishi R, Araki T (2008) Cloning of a novel gene encoding β-1,3-xylosidase from a marine bacterium, Vibrio sp. strain XY-214, and characterization of the gene product. Appl Environ Microbiol 74:305–308
Viborg AH, Sørensen KI, Gilad O, Steen-Jensen DB, Dilokpimol A, Jacobsen S, Svensson B (2013) Biochemical and kinetic characterisation of a novel xylooligosaccharide-upregulated GH43 β-D-xylosidase/α-L-arabinofuranosidase (BXA43) from the probiotic Bifidobacterium animalis subsp. lactis BB-12. AMB Express 3(1):56
Wagschal K, Heng C, Lee CC, Wong DW (2009) Biochemical characterization of a novel dual-function arabinofuranosidase/xylosidase isolated from a compost starter mixture. Appl Microbiol Biotechnol 81(5):855–863
Wagschal K, Jordan B, Braker JD (2012) Catalytic properties of β-D-xylosidase XylBH43 from Bacillus halodurans C-125 and mutant XylBH43-W147G. Process Biochem 47(3):366–372
Yang X, Shi P, Huang H, Luo H, Wang Y, Zhang W, Yao B (2014) Two xylose-tolerant GH43 bifunctional β-xylosidase/α-arabinosidases and one GH11 xylanase from Humicola insolens and their synergy in the degradation of xylan. Food Chem 148:381–387
Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y (2015) The I-TASSER suite: protein structure and function prediction. Nat Methods 12(1):7–8
Yeoman CJ, Han Y, Dodd D, Schroeder CM, Mackie RI, Cann IK (2010) Thermostable enzymes as biocatalysts in the biofuel industry. Adv Appl Microbiol 70:1–55
Acknowledgments
Authors thank MINCYT and CONICET for financial support. OO and FP hold post-doctoral and doctoral fellowships from the Argentinean National Council of Research (CONICET). SG holds an INTA doctoral fellowship. EC, PT, and MC acknowledge CONICET as Research Career members and RM belongs to National Institute of Agriculture Technology (INTA). Pre-treated wheat straw was gently supplied by Dr. Mercedes Ballesteros from CIEMAT, Spain. We thank Igor Polikarpov from USP, Brazil, for critical reading of the manuscript.
Funding
This study was funded by projects MINCYT PICT2014-0791 and CONICET PIP2015-11220150100121CO.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ornella Ontañon declares that she has no conflict of interest. Silvina Ghio declares that she has no conflict of interest. Rubén Marrero Díaz de Villegas declares that he has no conflict of interest. Florencia E Piccinni declares that she has no conflict of interest. Paola M Talia declares that she has no conflict of interest. María L Cerutti declares that she has no conflict of interest. Eleonora Campos declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors
Electronic supplementary material
ESM 1
(PDF 448 kb)
Rights and permissions
About this article
Cite this article
Ontañon, O.M., Ghio, S., Marrero Díaz de Villegas, R. et al. EcXyl43 β-xylosidase: molecular modeling, activity on natural and artificial substrates, and synergism with endoxylanases for lignocellulose deconstruction. Appl Microbiol Biotechnol 102, 6959–6971 (2018). https://doi.org/10.1007/s00253-018-9138-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9138-7