Abstract
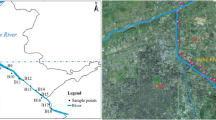
In many highly urbanized areas, effluent from wastewater treatment plants (WWTPs) represents a significant proportion of the water source for receiving rivers. Microbial communities are major components of riverine ecosystems and mediate the processes of nutrients and organic matter produced by treated and untreated WWTP effluent. To date, the impacts of WWTP effluent discharge on riverine microbial communities remain poorly understood. Based on 16S rRNA gene sequencing and water quality analysis, we investigated the microbial community compositions and predicted functions in the effluents of five municipal WWTPs and their receiving rivers. The results showed that the microbial compositions in the five WWTP effluents with different treatment processes were similar. Significant differences in the microbial community were not noted between the effluent, upstream, and downstream sites for both sampling months. However, dissimilarity of microbial composition between two sampling periods was observed. The temperature, pH, dissolved oxygen, and ammonium were major environmental factors associated with microbial community changes. Functional annotations of microbial communities based on 16S amplicons identified xenobiotic degradation and metabolism functions in effluent and river samples. Quantitative polymerase chain reaction revealed the dominance of ammonia-oxidizing bacteria (AOB) over ammonia-oxidizing archaea (AOA) in the WWTP effluents and rivers, and significant positive correlation between AOB abundance and nitrate concentration was observed. These findings will help increase our understanding of the impact of effluent discharge on urban river ecosystems.





Similar content being viewed by others
References
Arndt D, Xia JG, Liu YF, Zhou Y, Guo AC, Cruz JA, Sinelnikov I, Budwill K, Nesbo CL, Wishart DS (2012) METAGENassist: a comprehensive web server for comparative metagenomics. Nucleic Acids Res 40(W1):W88–W95
Asshauer KP, Wemheuer B, Daniel R, Meinicke P (2015) Tax4Fun: predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 31(17):2882–2884
Atashgahi S, Aydin R, Dimitrov MR, Sipkema D, Hamonts K, Lahti L, Maphosa F, Kruse T, Saccenti E, Springael D, Dejonghe W, Smidt H (2015) Impact of a wastewater treatment plant on microbial community composition and function in a hyporheic zone of a eutrophic river. Sci Rep-Uk 5:17284. doi:10.1038/Srep17284
Bai YH, Sun QH, Wen DH, Tang XY (2012) Abundance of ammonia-oxidizing bacteria and archaea in industrial and domestic wastewater treatment systems. FEMS Microbiol Ecol 80(2):323–330
Bai YH, Qi WX, Liang JS, Qu JH (2014) Using high-throughput sequencing to assess the impacts of treated and untreated wastewater discharge on prokaryotic communities in an urban river. Appl Microbiol Biot 98(4):1841–1851
Brooks BW, Riley TM, Taylor RD (2006) Water quality of effluent-dominated ecosystems: ecotoxicological, hydrological, and management considerations. Hydrobiologia 556:365–379
Bunzel K, Kattwinkel M, Liess M (2013) Effects of organic pollutants from wastewater treatment plants on aquatic invertebrate communities. Water Res 47(2):597–606
Cai W, Li Y, Wang P, Niu L, Zhang W, Wang C (2016) Effect of the pollution level on the functional bacterial groups aiming at degrading bisphenol A and nonylphenol in natural biofilms of an urban river. Environ Sci Pollut Res. doi:10.1007/s11356-016-6757-3
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Tumbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7(5):335–336
Chao A (1987) Estimating the population-size for capture recapture data with unequal catchability. Biometrics 43(4):783–791
Crump BC, Peterson BJ, Raymond PA, Amon RMW, Rinehart A, McClelland JW, Holmes RM (2009) Circumpolar synchrony in big river bacterioplankton. P Natl Acad Sci USA 106(50):21208–21212
DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL (2006) Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microb 72(7):5069–5072
Drury B, Rosi-Marshall E, Kelly JJ (2013) Wastewater treatment effluent reduces the abundance and diversity of benthic bacterial communities in urban and suburban rivers. Appl Environ Microb 79(6):1897–1905
Dyer SD, Peng C, McAvoy DC, Fendinger NJ, Masscheleyn P, Castillo LV, Lim JMU (2003) The influence of untreated wastewater to aquatic communities in the Balatuin River, The Philippines. Chemosphere 52(1):43–53
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26(19):2460–2461
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat methods 10(10):996−998
Fisher JC, Levican A, Figueras MJ, McLellan SL (2014) Population dynamics and ecology of Arcobacter in sewage. Front Microbiol 5:525. doi:10.3389/fmicb.2014.00525
Gao J, Luo X, Wu G, Li T, Peng Y (2014) Abundance and diversity based on amoA genes of ammonia-oxidizing archaea and bacteria in ten wastewater treatment systems. Appl Microbiol Biot 98(7):3339–3354
Glejser H (1969) A new test for heteroskedasticity. J Am Stat Assoc 64(325):316–323
Goni-Urriza M, Capdepuy M, Raymond N, Quentin C, Caumette P (1999) Impact of an urban effluent on the bacterial community structure in the Arga River (Spain), with special reference to culturable gram-negative rods. Can J Microbiol 45(10):826–832
Gucker B, Brauns M, Pusch MT (2006) Effects of wastewater treatment plant discharge on ecosystem structure and function of lowland streams. J N Am Benthol Soc 25(2):313–329
Helbling DE, Johnson DR, Honti M, Fenner K (2012) Micropollutant biotransformation kinetics associate with WWTP process parameters and microbial community characteristics. Environ Sci Technol 46(19):10579–10588
Langille MGI, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C (2013) Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol 31(9):814–821
Lee K-H, Wang Y-F, Wang Y, Gu J-D, Jiao JJ (2016) Abundance and diversity of aerobic/anaerobic ammonia/ammonium-oxidizing microorganisms in an ammonium-rich aquitard in the Pearl River Delta of South China. Microb Ecol:1–11
Li D, Sharp JO, Drewes JE (2016) Influence of wastewater discharge on the metabolic potential of the microbial community in river sediments. Microb Ecol 71(1):78–86
Logue JB, Stedmon CA, Kellerman AM, Nielsen NJ, Andersson AF, Laudon H, Lindström ES, Kritzberg ES (2016) Experimental insights into the importance of aquatic bacterial community composition to the degradation of dissolved organic matter. The ISME Journal 10(3):533–545
Lu X-M, Chen C, Zheng T-L (2016) Metagenomic insights into effects of chemical pollutants on microbial community composition and function in estuarine sediments receiving polluted river water. Microb Ecol:1–10
Magalhães CM, Machado A, Bordalo AA (2009) Temporal variability in the abundance of ammonia-oxidizing bacteria vs. archaea in sandy sediments of the Douro River estuary, Portugal. Aquat Microb Ecol 56(1):13–23
Marti E, Balcazar JL (2014) Use of pyrosequencing to explore the benthic bacterial community structure in a river impacted by wastewater treatment plant discharges. Res Microbiol 165(6):468–471
McClain ME, Boyer EW, Dent CL, Gergel SE, Grimm NB, Groffman PM, Hart SC, Harvey JW, Johnston CA, Mayorga E, McDowell WH, Pinay G (2003) Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6(4):301–312
Mosier AC, Francis CA (2008) Relative abundance and diversity of ammonia-oxidizing archaea and bacteria in the San Francisco Bay estuary. Environ Microbiol 10(11):3002–3016
Noble RT, Fuhrman JA (1998) Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquat Microb Ecol 14(2):113–118
Parks DH, Tyson GW, Hugenholtz P, Beiko RG (2014) STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30(21):3123–3124
Pernet-Coudrier B, Qi WX, Liu HJ, Muller B, Berg M (2012) Sources and pathways of nutrients in the semi-arid region of Beijing Tianjin, China. Environ Sci Technol 46(10):5294–5301
Prat N, Rieradevall M, Barata C, Munne A (2013) The combined use of metrics of biological quality and biomarkers to detect the effects of reclaimed water on macroinvertebrate assemblages in the lower part of a polluted Mediterranean river (Llobregat River, NE Spain). Ecol Indic 24:167–176
Pylro VS, Roesch LFW, Morais DK, Clark IM, Hirsch PR, Tótola MR (2014) Data analysis for 16S microbial profiling from different benchtop sequencing platforms. J Microbiol Methods 107:30–37
Qi WX, Singer H, Berg M, Muller B, Pernet-Coudrier B, Liu HJ, Qu JH (2015) Elimination of polar micropollutants and anthropogenic markers by wastewater treatment in Beijing, China. Chemosphere 119:1054–1061
Riedinger-Whitmore MA, Whitmore TJ, Smoak JM, Brenner M, Moore A, Curtis J, Schelske CL (2005) Cyanobacterial proliferation is a recent response to eutrophication in many Florida lakes: a paleolimnological assessment. Lake Reserv Manage 21(4):423–435
Santoro AE, Francis CA, De Sieyes NR, Boehm AB (2008) Shifts in the relative abundance of ammonia-oxidizing bacteria and archaea across physicochemical gradients in a subterranean estuary. Environ Microbiol 10(4):1068–1079
Schwarzenbach RP, Escher BI, Fenner K, Hofstetter TB, Johnson CA, von Gunten U, Wehrli B (2006) The challenge of micropollutants in aquatic systems. Science 313(5790):1072–1077
Sergeant MJ, Constantinidou C, Cogan T, Penn CW, Pallen MJ (2012) High-throughput sequencing of 16S rRNA gene amplicons: effects of extraction procedure, primer length and annealing temperature. PLoS One 7(5):e38094
Sonthiphand P, Cejudo E, Schiff SL, Neufeld JD (2013) Wastewater effluent impacts ammonia-oxidizing prokaryotes of the Grand River, Canada. Appl Environ Microb 79(23):7454–7465
Spanhoff B, Bischof R, Bohme A, Lorenz S, Neumeister K, Nothlich A, Kusel K (2007) Assessing the impact of effluents from a modern wastewater treatment plant on breakdown of coarse particulate organic matter and benthic macroinvertebrates in a lowland river. Water Air Soil Poll 180(1–4):119–129
Tank JL, Rosi-Marshall EJ, Griffiths NA, Entrekin SA, Stephen ML (2010) A review of allochthonous organic matter dynamics and metabolism in streams. J N Am Benthol Soc 29(1):118–146
Thomas F, Hehemann J-H, Rebuffet E, Czjzek M, Michel G (2011) Environmental and gut Bacteroidetes: the food connection. Front Microbiol 2(93). doi:10.3389/fmicb.2011.00093
Waiser MJ, Tumber V, Holm J (2011) Effluent-dominated streams. Part 1: presence and effects of excess nitrogen and phosphorus in Wascana Creek, Saskatchewan. Canada Environ Toxicol Chem 30(2):496–507
Wakelin SA, Colloff MJ, Kookana RS (2008) Effect of wastewater treatment plant effluent on microbial function and community structure in the sediment of a freshwater stream with variable seasonal flow. Appl Environ Microb 74(9):2659–2668
Wankel SD, Mosier AC, Hansel CM, Paytan A, Francis CA (2011) Spatial variability in nitrification rates and ammonia-oxidizing microbial communities in the agriculturally impacted Elkhorn Slough Estuary, California. Appl Environ Microb 77(1):269–280
Wright IA, Chessman BC, Fairweather PG, Benson LJ (1995) Measuring the impact of sewage effluent on the macroinvertebrate community of an upland stream—the effect of different levels of taxonomic resolution and quantification. Aust J Ecol 20(1):142–149
Zhang Q, Tang F, Zhou Y, Xu J, Chen H, Wang M, Laanbroek HJ (2015) Shifts in the pelagic ammonia-oxidizing microbial communities along the eutrophic estuary of Yong River in Ningbo City, China. Front Microbiol 6:1180
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Funding No. 51420105012 and 51578537) and the Chinese Academy of sciences (ZDRW-ZS-2016-5-6). The authors thank the BGI Central China (Wuhan) for high-throughput sequencing services. We also kindly thank the other members of the EcoImprove team for their help in the sampling.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
(PDF 12113 kb)
Rights and permissions
About this article
Cite this article
Huo, Y., Bai, Y. & Qu, J. Unravelling riverine microbial communities under wastewater treatment plant effluent discharge in large urban areas. Appl Microbiol Biotechnol 101, 6755–6764 (2017). https://doi.org/10.1007/s00253-017-8384-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8384-4