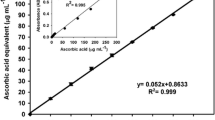
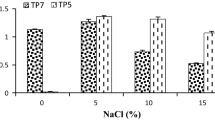
Abstract
Mesorhizobium alhagi, a legume-symbiont soil bacterium that forms nodules with the desert plant Alhagi sparsifolia, can produce large amounts of exopolysaccharide (EPS) using mannitol as carbon source. However, the role of EPS in M. alhagi CCNWXJ12-2T, an EPS-producing rhizobium with high salt resistance, remains uncharacterized. Here, we studied the role of EPS in M. alhagi CCNWXJ12-2T using EPS-deficient mutants constructed by transposon mutagenesis. The insertion sites of six EPS-deficient mutants were analyzed using single primer PCR, and two putative gene clusters were found to be involved in EPS synthesis. EPS was extracted and quantified, and EPS production in the EPS-deficient mutants was decreased by approximately 25 times compared with the wild-type strain. Phenotypic analysis revealed reduced salt resistance, antioxidant capacity, and cell motility of the mutants compared with the wild-type strain. In conclusion, our results indicate that EPS can influence cellular Na+ content and antioxidant enzyme activity, as well as play an important role in the stress adaption and cell motility of M. alhagi CCNWXJ12-2T.








Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215(3):403–410
Banjerdkij P, Vattanaviboon P, Mongkolsuk S (2005) Exposure to cadmium elevates expression of genes in the OxyR and OhrR regulons and induces cross-resistance to peroxide killing treatment in Xanthomonas campestris. Appl Environ Microb 71(4):1843–1849. doi:10.1128/Aem.71.4.1843-1849.2005
Battisti L, Lara JC, Leigh JA (1992) Specific oligosaccharide form of the Rhizobium meliloti exopolysaccharide promotes nodule invasion in alfalfa. P Natl Acad Sci USA 89(12):5625–5629. doi:10.1073/pnas.89.12.5625
Becker A, Kleickmann A, Keller M, Arnold W, Puhler A (1993) Identification and analysis of the Rhizobium meliloti exoAMONP genes involved in exopolysaccharide biosynthesis and mapping of promoters located on the exoHKLAMONP fragment. Mol Gen Genet 241(3–4):367–379
Branda SS, Vik A, Friedman L, Kolter R (2005) Biofilms: the matrix revisited. Trends Microbiol 13(1):20–26. doi:10.1016/j.tim.2004.11.006
Castellane TCL, Persona MR, Campanharo JC, Lemos EGD (2015) Production of exopolysaccharide from rhizobia with potential biotechnological and bioremediation applications. Int J Biol Macromol 74:515–522. doi:10.1016/j.ijbiomac.2015.01.007
Chang WS, Park KM, Koh SC, So JS (2008) Characterization of the Bradyrhizobium japonicum galE gene: its impact on lipopolysaccharide profile and nodulation of soybean. FEMS Microbiol Lett 280(2):242–249. doi:10.1111/j.1574-6968.2008.01066.x
Chen WM, Zhu WF, Bontemps C, Young JP, Wei GH (2010) Mesorhizobium alhagi sp. nov., isolated from wild Alhagi sparsifolia in North-Western China. Int J Syst Evol Microbiol 60(Pt 4):958–962. doi:10.1099/ijs.0.014043-0
Daniels R, Reynaert S, Hoekstra H, Verreth C, Janssens J, Braeken K, Fauvart M, Beullens S, Heusdens C, Lambrichts I, De Vos DE, Vanderleyden J, Vermant J, Michiels J (2006) Quorum signal molecules as biosurfactants affecting swarming in Rhizobium etli. P Natl Acad Sci USA 103(40):14965–14970. doi:10.1073/pnas.0511037103
Deaker R, Roughley RJ, Kennedy IR (2004) Legume seed inoculation technology - a review. Soil Biol Biochem 36(8):1275–1288. doi:10.1016/j.soilbio.2004.04.009
Degeest B, de Vuyst L (2000) Correlation of activities of the enzymes alpha-phosphoglucomutase, UDP-galactose 4-epimerase, and UDP-glucose pyrophosphorylase with exopolysaccharide biosynthesis by Streptococcus thermophilus LY03. Appl Environ Microbiol 66(8):3519–3527
Dhooghe I, Michiels J, Vlassak K, Verreth C, Waelkens F, Vanderleyden J (1995) Structural and functional analysis of the fixLJ genes of Rhizobium leguminosarum biovar phaseoli CNPAF512. Mol Gen Genet 249(1):117–126. doi:10.1007/Bf00290243
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Flemming HC (1993) Biofilms and environmental protection. Water Sci Technol 27(7–8):1–10
Garrison-Schilling KL, Kaluskar ZM, Lambert B, Pettis GS (2014) Genetic analysis and prevalence studies of the brp exopolysaccharide locus of Vibrio vulnificus. PLoS One 9(7):e100890. doi:10.1371/journal.pone.0100890
Harrison A, Bakaletz LO, Munson RS Jr (2012) Haemophilus influenzae and oxidative stress. Front Cell Infect Mi 2:40. doi:10.3389/fcimb.2012.00040
Janczarek M (2011) Environmental signals and regulatory pathways that influence exopolysaccharide production in rhizobia. Int J Mol Sci 12(11):7898–7933. doi:10.3390/ijms12117898
Janczarek M, Rachwal K (2013) Mutation in the pssA Gene involved in exopolysaccharide synthesis leads to several physiological and symbiotic defects in Rhizobium leguminosarum bv. trifolii. Int J Mol Sci 14(12):23711–23735. doi:10.3390/ijms141223711
Janczarek M, Urbanik-Sypniewska T (2013) Expression of the Rhizobium leguminosarum bv. trifolii pssA Gene, involved in exopolysaccharide synthesis, is regulated by RosR, phosphate, and the carbon source. J Bacteriol 195(15):3412–3423. doi:10.1128/Jb.02213-12
Janczarek M, Rachwal K, Ciesla J, Ginalska G, Bieganowski A (2015) Production of exopolysaccharide by Rhizobium leguminosarum bv. trifolii and its role in bacterial attachment and surface properties. Plant Soil 388(1–2):211–227. doi:10.1007/s11104-014-2320-5
Jaszek M, Janczarek M, Kuczynski K, Piersiak T, Grzywnowicz K (2014) The response of the Rhizobium leguminosarum bv. trifolii wild-type and exopolysaccharide-deficient mutants to oxidative stress. Plant Soil 376(1–2):75–94. doi:10.1007/s11104-013-1959-7
Jofré E, Becker A (2009) Production of succinoglycan polymer in Sinorhizobium meliloti is affected by SMb21506 and requires the N-terminal domain of ExoP. Mol Plant Microbe In 22(12):1656–1668. doi:10.1094/Mpmi-22-12-1656
Karlyshev AV, Pallen MJ, Wren BW (2000) Single-primer PCR procedure for rapid identification of transposon insertion sites. BioTechniques 28(6):1078
Kim YJ, Moon MH, Song JY, Smith CP, Hong SK, Chang YK (2008) Acidic pH shock induces the expressions of a wide range of stress-response genes. BMC Genomics 9:604. doi:10.1186/1471-2164-9-604
Kohler T, Curty LK, Barja F, van Delden C, Pechere JC (2000) Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J Bacteriol 182(21):5990–5996. doi:10.1128/Jb.182.21.5990-5996.2000
Kucuk C, Kivanc M (2009) Extracellular polysaccharide production by Rhizobium ciceri from Turkey. Ann Microbiol 59(1):141–144
Larsen RA, Wilson MM, Guss AM, Metcalf WW (2002) Genetic analysis of pigment biosynthesis in Xanthobacter autotrophicus Py2 using a new, highly efficient transposon mutagenesis system that is functional in a wide variety of bacteria. Arch Microbiol 178(3):193–201. doi:10.1007/s00203-002-0442-2
Lehman AP, Long SR (2013) Exopolysaccharides from Sinorhizobium meliloti can protect against H2O2-dependent damage. J Bacteriol 195(23):5362–5369. doi:10.1128/jb.00681-13
Leigh JA, Walker GC (1994) Exopolysaccharides of Rhizobium: synthesis, regulation and symbiotic function. Trends Genet 10(2):63–67
Li CT, Liao CT, Du SC, Hsiao YP, Lo HH, Hsiao YM (2014) Functional characterization and transcriptional analysis of galE gene encoding a UDP-galactose 4-epimerase in Xanthomonas campestris pv. campestris. Microbiol Res 169(5–6):441–452. doi:10.1016/j.micres.2013.08.005
Liu X, Luo Y, Mohamed OA, Liu D, Wei G (2014) Global transcriptome analysis of Mesorhizobium alhagi CCNWXJ12-2 under salt stress. BMC Microbiol 14:1. doi:10.1186/s12866-014-0319-y
Liu X, Luo Y, Li Z, Wei G (2016) Functional analysis of PrkA - a putative serine protein kinase from Mesorhizobium alhagi CCNWXJ12-2 - in stress resistance. BMC Microbiol 16(1):227. doi:10.1186/s12866-016-0849-6
Mansharamani M, Hewetson A, Chilton BS (2001) Cloning and characterization of an atypical type IV P-type ATPase that binds to the RING motif of RUSH transcription factors. J Biol Chem 276(5):3641–3649. doi:10.1074/jbc.M004231200
Mendis HC, Queiroux C, Brewer TE, Davis OM, Washburn BK, Jones KM (2013) The succinoglycan endoglycanase encoded by exoK is required for efficient Symbiosis of Sinorhizobium meliloti 1021 with the host plants Medicago truncatula and Medicago sativa (alfalfa). Mol Plant Microbe In 26(9):1089–1105. doi:10.1094/Mpmi-03-13-0087-R
Mols M, Abee T (2011) Primary and secondary oxidative stress in Bacillus. Environ Microbiol 13(6):1387–1394. doi:10.1111/j.1462-2920.2011.02433.x
Mols M, van Kranenburg R, van Melis CC, Moezelaar R, Abee T (2010) Analysis of acid-stressed Bacillus cereus reveals a major oxidative response and inactivation-associated radical formation. Environ Microbiol 12(4):873–885. doi:10.1111/j.1462-2920.2009.02132.x
Mozzi F, Savoy de Giori G, Font de Valdez G (2003) UDP-galactose 4-epimerase: a key enzyme in exopolysaccharide formation by Lactobacillus casei CRL 87 in controlled pH batch cultures. J Appl Microbiol 94(2):175–183
Navarini L, Cesaro A, Rossmurphy SB (1992) Exopolysaccharides from Rhizobium meliloti ye-2 grown under different Osmolarity conditions - viscoelastic properties. Carbohyd Res 223:227–234. doi:10.1016/0008-6215(92)80019-W
Neidhardt FC (1987) Escherichia coli And Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington DC
Nogales J, Bernabeu-Roda L, Cuellar V, Soto MJ (2012) ExpR is not required for swarming but promotes sliding in Sinorhizobium meliloti. J Bacteriol 194(8):2027–2035. doi:10.1128/jb.06524-11
Ophir T, Gutnick DL (1994) A role for exopolysaccharides in the protection of microorganisms from desiccation. Appl Environ Microb 60(2):740–745
Priyanka P, Arun AB, Ashwini P, Rekha PD (2015) Versatile properties of an exopolysaccharide R-PS18 produced by Rhizobium sp. PRIM-18. Carbohyd Polym 126:215–221. doi:10.1016/j.carbpol.2015.03.017
Rinaudi LV, Gonzalez JE (2009) The low-molecular-weight fraction of exopolysaccharide II from Sinorhizobium meliloti is a crucial determinant of biofilm formation. J Bacteriol 191(23):7216–7224. doi:10.1128/Jb.01063-09
Rinaudi LV, Sorroche F, Zorreguieta Á, Giordano W (2010) Analysis of the mucR gene regulating biosynthesis of exopolysaccharides: implications for biofilm formation in Sinorhizobium meliloti Rm1021. FEMS Microbiol Lett 302(1):15–21. doi:10.1111/j.1574-6968.2009.01826.x
Rodriguez-Navarro DN, Rodriguez-Carvajal MA, Acosta-Jurado S, Soto MJ, Margaret I, Crespo-Rivas JC, Sanjuan J, Temprano F, Gil-Serrano A, Ruiz-Sainz JE, Vinardell JM (2014) Structure and biological roles of Sinorhizobium fredii HH103 exopolysaccharide. PLoS One 9(12):e115391. doi:10.1371/journal.pone.0115391
Sandal I, Inzana TJ, Molinaro A, De Castro C, Shao JQ, Apicella MA, Cox AD, St Michael F, Berg G (2011) Identification, structure, and characterization of an exopolysaccharide produced by Histophilus somni during biofilm formation. BMC Microbiol 11:186. doi:10.1186/1471-2180-11-186
Staudt AK, Wolfe LG, Shrout JD (2012) Variations in exopolysaccharide production by Rhizobium tropici. Arch Microbiol 194(3):197–206. doi:10.1007/s00203-011-0742-5
Sule S, Cursino L, Zheng D, Hoch HC, Burr TJ (2009) Surface motility and associated surfactant production in Agrobacterium vitis. Lett Appl Microbiol 49(5):596–601. doi:10.1111/j.1472-765X.2009.02716.x
Urzainqui A, Walker GC (1992) Exogenous suppression of the symbiotic deficiencies of Rhizobium meliloti exo mutants. J Bacteriol 174(10):3403–3406
Vriezen JAC, de Bruijn FJ, Nusslein K (2007) Responses of rhizobia to desiccation in relation to osmotic stress, oxygen, and temperature. Appl Environ Microb 73(11):3451–3459. doi:10.1128/Aem.02991-06
Wang P, Zhong ZT, Zhou J, Cai T, Zhu J (2008) Exopolysaccharide biosynthesis is important for Mesorhizobium tianshanense: plant host interaction. Arch Microbiol 189(5):525–530. doi:10.1007/s00203-007-0345-3
Wilson T, Carson J (2001) Rapid, high-throughput extraction of bacterial genomic DNA from selective-enrichment culture media. Lett Appl Microbiol 32(5):326–330
Ye SH, Zhang MP, Yang H, Wang H, Xiao S, Liu Y, Wang JH (2014) Biosorption of Cu2+, Pb2+ and Cr6+ by a novel exopolysaccharide from Arthrobacter ps-5. Carbohyd Polym 101:50–56. doi:10.1016/j.carbpol.2013.09.021
York GM, Walker GC (1997) The Rhizobium meliloti exoK gene and prsD/prsE/exsH genes are components of independent degradative pathways which contribute to production of low-molecular-weight succinoglycan. Mol Microbiol 25(1):117–134. doi:10.1046/j.1365-2958.1997.4481804.x
York GM, Walker GC (1998a) The Rhizobium meliloti ExoK and ExsH glycanases specifically depolymerize nascent succinoglycan chains. P Natl Acad Sci USA 95(9):4912–4917. doi:10.1073/pnas.95.9.4912
York GM, Walker GC (1998b) The succinyl and acetyl modifications of succinoglycan influence susceptibility of succinoglycan to cleavage by the Rhizobium meliloti glycanases ExoK and ExsH. J Bacteriol 180(16):4184–4191
Zeng FJ, Zhang XM, Foetzki A, Li XY, Li XM, Runge M (2002) Water relation characteristics of Alhagi sparsifolia and consequences for a sustainable management. Sci China Ser D 45:125–131. doi:10.1007/Bf02878398
Zhou ML, Chen WM, Chen HY, Wei GH (2012) Draft genome sequence of Mesorhizobium alhagi CCNWXJ12-2(tau), a novel salt-resistant species isolated from the desert of northwestern China. J Bacteriol 194(5):1261–1262. doi:10.1128/Jb.06635-11
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by the National Key Research & Development Program (2016YFD0200308) and National Natural Science Foundation of China (41671261, 31370142, 31270012).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Electronic supplementary material
ESM 1
Table S1 Primers used in this study (PDF 269 kb)
Rights and permissions
About this article
Cite this article
Liu, X., Luo, Y., Li, Z. et al. Role of exopolysaccharide in salt stress resistance and cell motility of Mesorhizobium alhagi CCNWXJ12–2T . Appl Microbiol Biotechnol 101, 2967–2978 (2017). https://doi.org/10.1007/s00253-017-8114-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-017-8114-y