Abstract
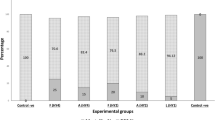
Riemerella anatipestifer infection causes high mortality for ducks which results in major economic losses in the duck industry. In this study, we identified a mutant strain RA-M1 by Tn4351 transposon mutagenesis, in which the M949_1603 gene encoding glycosyl transferase was inactivated. PCR analysis revealed that M949_1603 gene is specifically existed in R. anatipestifer serotype 1 strains. RA-M1 presented no reactivity to the anti-lipopolysaccharide (LPS) MAb in an indirect ELISA. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) followed by Western blotting demonstrated that RA-M1 LPS had a deficiency in ladder-like binding pattern to rabbit antiserum against R. anatipestifer serotype 1 strain CH3, indicating that the O-antigen structure of RA-M1 was changed. RA-M1 showed significant attenuated virulence in ducks and higher sensitivity to normal duck serum, compared with its parent strain CH3. Furthermore, cross-protection of RA-M1 for R. anatipestifer serotypes 1, 2, and 10 strains was evaluated. Ducks that received two immunizations with inactivated RA-M1 vaccine were 100 % protected from challenge with R. anatipestifer serotype 1 strain WJ4, serotype 2 strain Yb2, and serotype 10 strain HXb2. No changes were observed in the liver, heart, or spleen samples from the protected ducks during autopsy and histological examination. Furthermore, vaccination generated high antibody titers of 1:12,800 against serotypes 1, 2, and 10 strains and enhanced production of interleukin 2 (IL-2) and IL-4 in ducks. These results suggested that M949_1603 gene is associated with serotype 1 O-antigen biosynthesis, and mutant RA-M1 could be used as a novel cross-protection vaccine candidate to protect ducks against R. anatipestifer infection.






Similar content being viewed by others
References
Aquilini E, Merino S, Regue M, Tomas JM (2014) Genomic and proteomic studies on Plesiomanas shigelloides lipopolysaccharide core biosynthesis. J Bacteriol 196(3):556–567
Bagdasarian M, Lurz R, Ruckert B, Franklin FC, Bagdasarian MM, Frey J, Timmis KN (1981) Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene 16(1-3):237–247
Brown DB, Forsberg LS, Kannenberg EL, Carlson RW (2012) Characterization of galacturonosyl transferase genes rgtA, rgtB, rgtC, rgtD and rgtE responsible for lipopolysaccharide synthesis in nitrogen-fixing endosymbiont Rhizobium leguminosarum: lipopolysaccharide core and lipid galacturonosyl residues confer membrane stability. J.Biochem 287(2):935–949
Campagnari AA, Spinola SM, Lesse AJ, Kwaik YA, Mandrell RE, Apicella MA (1990) Lipooligosaccharide epitopes shared among gram-negative non-enteric mucosal pathogens. Microb Pathog 8(5):353–362
Fomsgaard A, Freudenberg M, Galanos C (1990) Modification of the silver staining technique to detect lipopolysaccharide in polyacrylamide gels. J Clin Microbiol 28(12):2627–2631
Grozdanov L, Zahringer U, Blum-Oehler G, Brade L, Henne A, Knirel Ya, Schombel U, Schulze J, Sonnenborn U, Gottschalk G, Hacker J, Rietschel ET, Dobrindt U (2002) A single nucleotide exchange in the wzy gene is responsible for the semirough O6 lipopolysaccharide phenotype and serum sensitivity of Escherichia coli strain Nissle 1917. J Bacteriol 184(21):5912–5925
Gu X-X, Chen J, Barenkamp SJ, Robbins JB, Tsai C-M, Lim DJ, Battey J (1998) Synthesis and characterization of lipooligosaccharide-based conjugates as vaccine candidates for Moraxella (Branhamella) catarrhalis. Infect Immun 66(5):1891–1897
Helfer DH, Helmboldt CF (1977) Pasteurella anatipestifer infection in turkeys. Avian Dis 21(4):712–715
Hu Q, Chen H, Liu X, Zhan M, Zhang Z, Deen S, Zhang Y (2002) Determination of growth curve of Riemerella anatipestifer. Chin Anim Husb Vet Med 34:8–9
Hu Q, Han X, Zhou X, Ding S, Ding C, Yu S (2010) Characterization of biofilm formation by Riemerella anatipestifer. Vet Microbiol 144(3):429–436
Hu Q, Han X, Zhou X, Ding C, Zhu Y, Yu S (2011) OmpA is a virulence factor of Riemerella anatipestifer. Vet Microbiol 150(3):278–283
Hu Q, Zhu Y, Tu J, Yin Y, Wang X, Han X, Ding C, Zhang B, Yu S (2012) Identification of the genes involved in Riemerella anatipestifer biofilm formation by random transposon mutagenesis. PLoS ONE 7(6) doi:10.1371/journal.pone.0039805
Liu H, Wang X, Ding C, Han X, Cheng A, Wang S, Yu S (2013) Development and evaluation of a trivalent Riemerella anatipestifer-inactivated vaccine. Clin Vaccine Immunol 20(5):691–697
Loh H, Teo T, Tan HC (1992) Serotypes of Pasteurella anatipestifer isolates from ducks in Singapore: a proposal of new serotypes. Avian Pathol 21(3):453–459
McQuillen DP, Gulati S, Rice PA (1994) Complement-mediated bacterial killing assays. Methods Enzymol 236:137–147
Nikaido H, Vaara M (1985) Molecular basis of bacterial outer membrane permeability. Microbiol Rev 49(1):1–32
Orr MT, Plessner Windish H, Beebe EA, Argilla D, Huang PW, Reese VA, Reed SG, Coler RN (2015) IFN-gamma and TNF are not essential parameters of CD4 T cell responses for vaccine control of tuberculosis. J Infect Dis. doi:10.1093/infdis/jiv055
Pathanasophon P, Sawada T, Tanticharoenyos T (1995) New serotypes of Riemerella anatipestifer isolated from ducks in Thailand. Avian Pathol 24(1):195–199
Pathanasophon P, Phuektes P, Tanticharoenyos T, Narongsak W, Sawada T (2002) A potential new serotype of Riemerella anatipestifer isolated from ducks in Thailand. Avian Pathol 31(3):267–270
Pei J, Ficht TA (2004) Brucella abortus rough mutants are cytopathic for macrophages in culture. Infect Immun 72(1):440–450
Perepelov AV, Wang Q, Levina EA, Ovchinnikova OG, Qian Y, Shashkov AS, Wang L, Knirel YA (2014) Structure and gene cluster of the O-antigen of Escherichia coli O36. Carbohydr Res 390:46–49
Pierce RL, Vorhies MW (1973) Pasteurella anatipestifer infection in geese. Avian Dis 17(4):868–870
Reed LJ, Muench H (1938) A simple method of estimating fifty per cent endpoints. Am J Epidemiol 27(3):493–497
Sandhu TS, Rimler R (1997) Riemerella anatipestifer infection. Diseases of Poultry 161–166
Segers P, Mannheim W, Vancanneyt M, De Brandt K, Hinz K-H, Kersters K, Vandamme P (1993) Riemerella anatipestifer gen. nov., comb. nov., the causative agent of septicemia anserum exsudativa, and its phylogenetic affiliation within the Flavobacterium-Cytophaga rRNA homology group. Int J Syst Bacteriol 43(4):768–776
Smith P, Krohn RI, Hermanson G, Mallia A, Gartner F, Provenzano M, Fujimoto E, Goeke N, Olson B, Klenk D (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150(1):76–85
Wang X, Ding C, Wang S, Han X, Hou W, Yue J, Zou J, Yu S (2014) The AS87_04050 gene is involved in bacterial lipopolysaccharide biosynthesis and pathogenicity of Riemerella anatipestifer. PLoS ONE 9(10), e109962. doi:10.1371/journal.pone.0109962
Wang X, Ding C, Han X, Wang S, Yue J, Hou W, Cao S, Zou J, Yu S (2015) Complete genome sequence of Riemerella anatipestifer serotype 1 strain CH3. Genome Announc. doi:10.1128/genomeA.01594-14
Warburg O, Christian W (1942) Isolation and crystallization of enolase. Biochem Z 310:384–421
Zhou D, Utkina N, Li D, Dong C, Druzhinina T, Veselovsky V, Liu B (2013) Biochemical characterization of a new beta-1,3-galactosyltransferase WbuP from Escherichia coli O114 that catalyzes the second step in O-antigen repeating-unit. Carbohydr Res 381:43–50. doi:10.1016/j.carres.2013.08.021
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31272591).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(PDF 10 kb)
Rights and permissions
About this article
Cite this article
Zou, J., Wang, X., Ding, C. et al. Characterization and cross-protection evaluation of M949_1603 gene deletion Riemerella anatipestifer mutant RA-M1. Appl Microbiol Biotechnol 99, 10107–10116 (2015). https://doi.org/10.1007/s00253-015-6848-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6848-y