Abstract
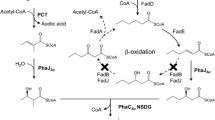
A polyhydroxyalkanoate (PHA) was enzymatically synthesized in vitro, and the end structure of PHA associated with a chain transfer (CT) reaction was investigated. In the CT reaction, PHA chain transfers from PHA synthase (PhaC) to a CT agent, resulting in covalent bonding of CT agent to the PHA chain at its carboxyl end. In vitro CT reaction has never been demonstrated because of relatively low yields of in vitro synthesized poly[(R)-3-hydroxybutyrate)] (P(3HB)), which makes it difficult to characterize the end structures of the polymers by nuclear magnetic resonance (NMR). To overcome these difficulties, a novel in vitro synthesis method that produced relatively larger amounts of P(3HB) was developed by employing PhaCDa from Delftia acidovorans and two enantioselective enoyl-coenzyme A (CoA) hydratases which were R-hydratase (PhaJAc) from Aeromonas caviae and S-hydratase (FadB1x) from Pseudomonas putida KT2440 with β-butyrolactone and CoA as starting materials. Using this method, P(3HB) synthesis was performed with tetraethylene glycols (TEGs) as a discriminable CT agent, and the resultant P(3HB) was characterized by 1H-NMR. NMR analysis revealed that the carboxylic end of P(3HB) was covalently linked to TEGs, providing the first direct evidence of in vitro CT reaction.



Similar content being viewed by others
References
Agus J, Kahar P, Abe H, Doi Y, Tsuge T (2006) Molecular weight characterization of poly[(R)-3-hydroxybutyrate] synthesized by genetically engineered strains of Escherichia coli. Polym Degrad Stab 91:1138–1146
Ashby R, Shi FY, Gross RA (1997) Use of poly(ethylene glycol) to control the end group structure and molecular weight of poly(3-hydroxybutyrate) formed by Alcaligenes latus DSM 1122. Tetrahedron 53:15209–15223
Fong JC, Schulz H (1981) Short-chain and long-chain enoyl-CoA hydratases from pig heart muscle. Methods Enzymol 71:390–398
Foster LJ (2007) Biosynthesis, properties and potential of natural–synthetic hybrids of polyhydroxyalkanoates and polyethylene glycols. Appl Microbiol Biotechnol 75:1241–1247
Fukui T, Shiomi N, Doi Y (1998) Expression and characterization of (R)-specific enoyl coenzyme A hydratase involved in polyhydroxyalkanoate biosynthesis by Aeromonas caviae. J Bacteriol 180:667–673
Gerngross TU, Martin DP (1995) Enzyme-catalyzed synthesis of poly[(R)-(−)-3-hydroxybutyrate]: formation of macroscopic granules in vitro. Proc Natl Acad Sci U S A 92:6279–6283
Kawaguchi Y, Doi Y (1990) Structure of native poly(3-hydroxybutyrate) granules characterized by X-ray diffraction. FEMS Microbiol Lett 70:151–155
Kawaguchi Y, Doi Y (1992) Kinetics and mechanism of synthesis and degradation of poly(3-hydroxybutyrate) in Alcaligenes eutrophus. Macromolecules 25:2324–2329
King MT, Reiss PD (1985) Separation and measurement of short-chain coenzyme-A compounds in rat liver by reversed-phase high-performance liquid chromatography. Anal Biochem 146:173–179
King MT, Reiss PD, Cornell NW (1988) Determination of short-chain coenzyme A compounds by reversed-phase high-performance liquid chromatography. Methods Enzymol 166:70–79
Lawrence AG, Choi J, Rha C, Stubbe J, Sinskey AJ (2005) In vitro analysis of the chain termination reaction in the synthesis of poly-(R)-β-hydroxybutyrate by the class III synthase from Allochromatium vinosum. Biomacromolecules 6:2113–2119
Lenz RW, Farcet C, Dijkstra PJ, Goodwin S, Zhang S (1999) Extracellular polymerization of 3-hydroxyalkanoate monomers with the polymerase of Alcaligenes eutrophus. Int J Biol Macromol 25:55–60
Madden LA, Anderson AJ, Shah DT, Asrar J (1999) Chain termination in polyhydroxyalkanoate synthesis: involvement of exogenous hydroxy-compounds as chain transfer agents. Int J Biol Macromol 25:43–53
Moskowitz GJ, Merrick JM (1969) Metabolism of poly-β-hydroxybutyrate. II. Enzymic synthesis of d-(−)-β-hydroxybutyryl coenzyme A by an enoyl hydrase from Rhodospirillum rubrum. Biochemistry 8:2748–2755
Nelson KE, Weinel C, Paulsen IT, Dodson RJ, Hilbert H, Martins dos Santos VAP, Fouts DE, Gill SR, Pop M, Holmes M, Brinkac L, Pop M, Holmes M, Brinkac L, Beanan M, DeBoy RT, Daugherty S, Kolonay J, Madupu R, Nelson W, White O, Peterson J, Khouri H, Hance I, Chris Lee P, Holtzapple E, Scanlan D, Tran K, Moazzez A, Utterback T, Rizzo M, Lee K, Kosack D, Moestl D, Wedler H, Lauber J, Stjepandic D, Hoheisel J, Straetz M, Heim S, Kiewitz C, Eisen J, Timmis KN, Düsterhöft A, Tümmler B, Fraser CM (2002) Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ Microbiol 4:799–808
Normi YM, Hiraishi T, Taguchi S, Abe H, Sudesh K, Najimudin N, Doi Y (2005) Characterization and properties of G4X mutants of Ralstonia eutropha PHA synthase for poly(3-hydroxybutyrate) biosynthesis in Escherichia coli. Macromol Biosci 5:197–206
Olson AR, Miller RJ (1938) The mechanism of the aqueous hydrolysis of β-butyrolactone. J Am Chem Soc 60:2687–2692
Sanguanchaipaiwong V, Gabelish CL, Hook J, Scholz C, Foster LJR (2004) Biosynthesis of natural–synthetic hybrid copolymers: polyhydroxyoctanoate–diethylene glycol. Biomacromolecules 5:643–649
Sato S, Nomura CT, Abe H, Doi Y, Tsuge T (2007) Poly[(R)-3-hydroxybutyrate] formation in Escherichia coli from glucose through an enoyl-CoA hydratase-mediated pathway. J Biosci Bioeng 103:38–44
Sato S, Ono Y, Mochiyama Y, Sivaniah E, Kikkawa Y, Sudesh K, Hiraishi T, Doi Y, Abe H, Tsuge T (2008) Polyhydroxyalkanoate film formation and synthase activity during in vitro and in situ polymerization on hydrophobic surfaces. Biomacromolecules 9:2811–2818
Shi F, Ashby R, Gross RA (1996a) Use of poly(ethylene glycol)s to regulate poly(3-hydroxybutyrate) molecular weight during Alcaligenes eutrophus cultivations. Macromolecules 29:7753–7758
Shi F, Gross RA, Rutherford DR (1996b) Microbial polyester synthesis: effects of poly(ethylene glycol) on product composition, repeat unit sequence, and end group structure. Macromolecules 29:10–17
Simon EJ, Shemin D (1953) The preparation of S-succinyl coenzyme A. J Am Chem Soc 75:2520
Song JJ, Zhang S, Lenz RW, Goodwin S (2000) In vitro polymerization and copolymerization of 3-hydroxypropionyl–CoA with the PHB synthase from Ralstonia eutropha. Biomacromolecules 1:433–439
Stubbe J, Tian J (2003) Polyhydroxyalkanoate (PHA) homeostasis: the role of the PHA synthase. Nat Prod Rep 20:445–457
Sudesh K, Fukui T, Doi Y (1998) Genetic analysis of Comamonas acidovorans polyhydroxyalkanoate synthase and factors affecting the incorporation of 4-hydroxybutyrate monomer. Appl Environ Microbiol 64:3437–3443
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci 25:1503–1555
Tajima K, Satoh Y, Nakazawa K, Tannai H, Erata T, Munekata M, Kamachi M, Lenz RW (2004) Chemoenzymatic synthesis of poly(3-hydroxybutyrate) in a water–organic solvent two-phase system. Macromolecules 37:4544–4546
Tomizawa S, Saito Y, Hyakutake M, Nakamura Y, Abe H, Tsuge T (2010) Chain transfer reaction catalyzed by various polyhydroxyalkanoate synthases with poly(ethylene glycol) as an exogenous chain transfer agent. Appl Microbiol Biotechnol 87:1427–1435
Tomizawa S, Yoshioka M, Ushimaru K, Tsuge T (2012) Preparative synthesis of poly[(R)-3-hydroxybutyrate] monomer for enzymatic cell-free polymerization. Polym J 44:982–985
Tsuge T, Imazu S, Takase K, Taguchi S, Doi Y (2004) An extra large insertion in the polyhydroxyalkanoate synthase from Delftia acidovorans DS-17: its deletion effects and relation to cellular proteolysis. FEMS Microbiol Lett 231:77–83
Valentin H, Steinbüchel A (1994) Application of enzymatically synthesized short-chain-length hydroxy fatty acid coenzyme A thioesters for assay of polyhydroxyalkanoic acid synthases. Appl Microbiol Biotechnol 40:699–709
Zhang S, Yasuo T, Lenz RW, Goodwin S (2000) Kinetic and mechanistic characterization of the polyhydroxybutyrate synthase from Ralstonia eutropha. Biomacromolecules 1:244–251
Acknowledgments
This work was supported by a Grant-In-Aid for Scientific Research (KAKENHI 19681008) to T. Tsuge. S. Tomizawa was supported by a Grant-in-Aid for JSPS Fellows (Tokyo Institute of Technology G-COE Program: Education and Research Centre for Material Innovation).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tomizawa, S., Sato, S., Lan, J.CW. et al. In vitro evidence of chain transfer to tetraethylene glycols in enzymatic polymerization of polyhydroxyalkanoate. Appl Microbiol Biotechnol 97, 4821–4829 (2013). https://doi.org/10.1007/s00253-013-4798-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4798-9