Abstract
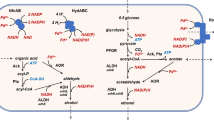
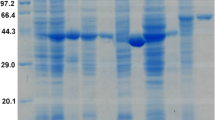
In this study, we identified and characterized mitochondrial alcohol dehydrogenase 3 from the thermotolerant methylotrophic yeast Hansenula polymorpha (HpADH3). The amino acid sequence of HpADH3 shares over 70% of its identity with the alcohol dehydrogenases of other yeasts and exhibits the highest similarity of 91% with the alcohol dehydrogenase 1 of H. polymorpha. However, unlike the cytosolic HpADH1, HpADH3 appears to be a mitochondrial enzyme, as a mitochondrial targeting extension exists at its N terminus. The recombinant HpADH3 overexpressed in Escherichia coli showed similar catalytic efficiencies for ethanol oxidation and acetaldehyde reduction. The HpADH3 displayed substrate specificities with clear preferences for medium chain length primary alcohols and acetaldehyde for an oxidation reaction and a reduction reaction, respectively. Although the H. polymorpha ADH3 gene was induced by ethanol in the culture medium, both an ADH isozyme pattern analysis and an ADH activity assay indicated that HpADH3 is not the major ADH in H. polymorpha DL-1. Moreover, HpADH3 deletion did not affect the cell growth on different carbon sources. However, when the HpADH3 mutant was complemented by an HpADH3 expression cassette fused to a strong constitutive promoter, the resulting strain produced a significantly increased amount of ethanol compared to the wild-type strain in a glucose medium. In contrast, in a xylose medium, the ethanol production was dramatically reduced in an HpADH3 overproduction strain compared to that in the wild-type strain. Taken together, our results suggest that the expression of HpADH3 would be an ideal engineering target to develop H. polymorpha as a substrate specific bioethanol production strain.





Similar content being viewed by others
References
Bakker BM, Bro C, Kotter P, Luttik MAH, van Dijken JP, Pronk JT (2000) The mitochondrial alcohol dehydrogenase Adh3p is involved in a redox shuttle in Saccharomyces cerevisiae. J Bacteriol 182:4730–4737
Blazhenko O, Zimmermann M, Kang H, Bartosz G, Penninckx M, Ubiyvovk V, Sibirny A (2006) Accumulation of cadmium ions in the methylotrophic yeast Hansenula polymorpha. BioMetals 19:593–599
Bozzi A, Saliola M, Falcone C, Bossa F, Martini F (1997) Structural and biochemical studies of alcohol dehydrogenase isozymes from Kluyveromyces lactis. Biochim Biophys Acta 1339:133–142
Claros MG, Vincens P (1996) Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem 241:779–786
Dmytruk O, Dmytruk K, Abbas C, Voronovsky A, Sibirny A (2008) Engineering of xylose reductase and overexpression of xylitol dehydrogenase and xylulokinase improves xylose alcoholic fermentation in the thermotolerant yeast Hansenula polymorpha. Microb Cell Fact 7:21
Eliasson A, Christensson C, Wahlbom CF, Hahn-Hagerdal B (2000) Anaerobic xylose fermentation by recombinant Saccharomyces cerevisiae carrying XYL1, XYL2, and XKS1 in mineral medium chemostat cultures. Appl Environ Microbiol 66:3381–3386
Fejér O, Orosz-Fejér K, Belea A (1979) Gel isoelectric focusing of wheat alcohol dehydrogenase. TAG Theor Appl Genet 54:37–39
Ganzhorn AJ, Green D, Hershey AD, Gould RM, Plapp BV (1987) Kinetic characterization of yeast alcohol dehydrogenases. Amino acid residue 294 and substrate specificity. J Biol Chem 262:3754–3761
Grabek-Lejko D, Kurylinko OO, Sibirny VA, Ubiyvovk VM, Penninckx M, Sibirny AA (2011) Alcoholic fermentation by wild-type Hansenula polymorpha and Saccharomyces cerevisiae versus recombinant strains with an elevated level of intracellular glutathione. J Ind Microbiol Biotechnol 38:1853–1859
Hartl F-U, Pfanner N, Nicholson DW, Neupert W (1989) Mitochondrial protein import. Biochim Biophys Acta 988:1–45
Hay R, Böhni P, Gasser S (1984) How mitochondria import proteins. Biochim Biophys Acta 779:65–87
Ho N, Chen Z, Brainard A, Sedlak M (1999) Successful design and development of genetically engineered Saccharomyces yeasts for effective cofermentation of glucose and xylose from cellulosic biomass to fuel ethanol. In: Tsao GT (ed) Recent progress in bioconversion of lignocellulosics. Springer, New York, pp 163–192
Ishchuk OP, Voronovsky AY, Stasyk OV, Gayda GZ, Gonchar MV, Abbas CA, Sibirny AA (2008) Overexpression of pyruvate decarboxylase in the yeast Hansenula polymorpha results in increased ethanol yield in high-temperature fermentation of xylose. FEMS Yeast Res 8:1164–1174
Kang HA, Kang W, Hong W-K, Kim MW, Kim J-Y, Sohn J-H, Choi E-S, Choe K-B, Rhee SK (2001) Development of expression systems for the production of recombinant human serum albumin using the MOX promoter in Hansenula polymorpha DL-1. Biotechnol Bioeng 76:175–185
Kang HA, Sohn J-H, Agaphonov MO, Choi E-S, Ter-Avanesyan MD, Rhee SK (2002) Development of expression systems for the production of recombinant proteins in Hansenula polymorpha DL-1. In: Gellissen G (ed) Hansenula polymorpha—biology and applications. Miley-VCH, Weinham, pp 124–146
Kim MW, Kim EJ, Kim JY, Park JS, Oh DB, Shimma Y, Chiba Y, Jigami Y, Rhee SK, Kang HA (2006) Functional characterization of the Hansenula polymorpha HOC1, OCH1, and OCR1 genes as members of the yeast OCH1 mannosyltransferase family involved in protein glycosylation. J Biol Chem 281:6261–6272
Leskovac V, Trivic S, Pericin D (2002) The three zinc-containing alcohol dehydrogenases from baker’s yeast, Saccharomyces cerevisiae. FEMS Yeast Res 2:481–494
Lyne R, Burns G, Mata J, Penkett C, Rustici G, Chen D, Langford C, Vetrie D, Bahler J (2003) Whole-genome microarrays of fission yeast: characteristics, accuracy, reproducibility, and processing of array data. BMC Genom 4:27
Mazzoni C, Iacchini S, Serafini A, Falcone C (2006) Characterization of a Kluyveromyces lactis mutant with altered regulation of mitochondrial alcohol dehydrogenases. FEMS Yeast Res 6:421–427
McAlister L, Holland MJ (1985) Differential expression of the three yeast glyceraldehydes-3-phosphate dehydrogenase genes. J Biol Chem 260:15019–15027
Neuhauser W, Haltrich D, Kulbe KD, Nidetzky B (1997) NAD(P)H-dependent aldose reductase from the xylose-assimilating yeast Candida tenuis. Isolation, characterization and biochemical properties of the enzyme. Biochem J 326:683–692
Olsson L, Hahn-Hagerdal B (1996) Fermentation of lignocellulosic hydrolysates for ethanol production. Enz Microbial Technol 18:312–331
Ozimek P, Lahtchev K, Kiel JAKW, Veenhuis M, Klei IJ (2004) Hansenula polymorpha Swi1p and Snf2p are essential for methanol utilisation. FEMS Yeast Res 4:673–682
Pilgrim D, Young ET (1987) Primary structure requirements for correct sorting of the yeast mitochondrial protein ADH III to the yeast mitochondrial matrix space. Mol Cell Biol 7:294–304
Postma E, Verduyn C, Scheffers WA, Van Dijken JP (1989) Enzymic analysis of the crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol 55:468–477
Reid MF, Fewson CA (1994) Molecular characterization of microbial alcohol dehydrogenases. Crit Rev Microbiol 20:13–56
Roa M, Blobel G (1983) Biosynthesis of peroxisomal enzymes in the methylotrophic yeast Hansenula polymorpha. Proc Nat’l Aca Sci U S A 80:6872–6876
Ryabova OB, Chmil OM, Sibirny AA (2003) Xylose and cellobiose fermentation to ethanol by the thermotolerant methylotrophic yeast Hansenula polymorpha. FEMS Yeast Res 4:157–164
Saliola M, Falcone C (1995) Two mitochondrial alcohol dehydrogenase activities of Kluyveromyces lactis are differently expressed during respiration and fermentation. Mol Gen Genet MGG 249:665–672
Saliola M, Maria ID, Lodi T, Fiori A, Falcone C (2006) KlADH3, a gene encoding a mitochondrial alcohol dehydrogenase, affects respiratory metabolism and cytochrome content in Kluyveromyces lactis. FEMS Yeast Res 6:1184–1192
Sambrook J, Russel DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor, Cold Spring Harbor Laboratory
Suwannarangsee S, Oh D-B, Seo J-W, Kim CH, Rhee SK, Kang HA, Chulalaksananukul W, Kwon O (2010) Characterization of alcohol dehydrogenase 1 of the thermotolerant methylotrophic yeast Hansenula polymorpha. Appl Microbiol Biotechnol 88:497–507
Verduyn C, Breedveld GJ, Scheffers WA, Van Dijken JP (1988) Substrate specificity of alcohol dehydrogenase from the yeast Hansenyls polymorpha CBS 4732 and Candida utilis CBS 621. Yeast 4:143–148
Voronovsky AY, Rohulya OV, Abbas CA, Sibirny AA (2009) Development of strains of the thermotolerant yeast Hansenula polymorpha capable of alcoholic fermentation of starch and xylan. Metab Eng 11:234–242
Wiedemann N, Frazier AE, Pfanner N (2004) The protein import machinery of mitochondria. J Biol Chem 279:14473–14476
Young ET, Pilgrim D (1985) Isolation and DNA sequence of ADH3, a nuclear gene encoding the mitochondrial isozyme of alcohol dehydrogenase in Saccharomyces cerevisiae. Mol Cell Biol 5:3024–3034
Yurimoto H, Lee B, Yasuda F, Sakai Y, Kato N (2004) Alcohol dehydrogenases that catalyse methyl formate synthesis participate in formaldehyde detoxification in the methylotrophic yeast Candida boidinii. Yeast 21:341–350
Acknowledgments
This work was supported by a grant from the KRIBB Research Initiative Program, by a Basic Science Research Program (no. 2009-0075186), and by a HTS-based Integrated Technology Development grant (no. 2008-2004174) through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology. S. Suwannarangsee was supported by the International Scholar Exchange Fellowship for the academic year of 2008–2009 by the Korea Foundation for Advanced Studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suwannarangsee, S., Kim, S., Kim, OC. et al. Characterization of alcohol dehydrogenase 3 of the thermotolerant methylotrophic yeast Hansenula polymorpha . Appl Microbiol Biotechnol 96, 697–709 (2012). https://doi.org/10.1007/s00253-011-3866-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3866-2