Abstract
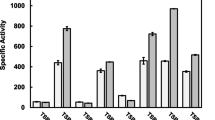
Four different β-galactosidases (previously named BbgI, BbgII, BbgIII and BbgIV) from Bifidobacterium bifidum NCIMB41171 were overexpressed in Escherichia coli, purified to homogeneity and their biochemical properties and substrate preferences comparatively analysed. BbgI was forming a hexameric protein complex of 875 kDa, whereas BbgII, BbgIII and BbgIV were dimers with native molecular masses of 178, 351 and 248 kDa, respectively. BbgII was the only enzyme that preferred acidic conditions for optimal activity (pH 5.4–5.8), whereas the other three exhibited optima in more neutral pH ranges (pH 6.4–6.8). Na+ and/or K+ ions were prerequisite for BbgI and BbgIV activity in Bis–Tris-buffered solutions, whereas Mg++ was strongly activating them in phosphate-buffered solutions. BbgII and BbgIII were slightly influenced from the presence or absence of cations, with Mg++, Mn++ and Ca++ ions exerting the most positive effect. Determination of the specificity constants (k cat/K m) clearly indicated that BbgI (6.11 × 104 s−1 M−1), BbgIII (2.36 × 104 s−1 M−1) and especially BbgIV (4.01 × 105 s−1 M−1) are highly specialised in the hydrolysis of lactose, whereas BbgII is more specific for β-d-(1→6) galactobiose (5.59 × 104 s−1 M−1) than lactose (1.48 × 103 s−1 M−1). Activity measurements towards other substrates (e.g. β-d-(1→6) galactobiose, β-d-(1→4) galactobiose, β-d-(1→4) galactosyllactose, N-acetyllactosamine, etc.) indicated that the β-galactosidases were complementary to each other by hydrolysing different substrates and thus contributing in a different way to the bacterial physiology.


Similar content being viewed by others
References
Biavati B, Mattarelli P (2001) The family Bifidobacteriaceae. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (eds) The prokaryotes. Springer, New York, pp 1–70
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 72:248–254
Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT (1987) Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28:1221–1227
Dionex Corporation (2001) Determination of trans-galactooligosaccharides in foods by AOAC method 2001.02. Application Note 155. Dionex Corporation, Sunnyvale, CA
Dumortier V, Brassart C, Bouquelet C (1994) Purification and properties of a β-d-galactosidase from Bifidobacterium bifidum exhibiting a transgalactosylation reaction. Biotech Appl Biochem 19:341–354
Goulas AK, Tzortzis G, Gibson GR (2007a) Development of a process for the production and purification of α- and β-galactooligosaccharides from Bifidobacterium bifidum NCIMB41171. Int Dairy J 17:648–656
Goulas TK, Goulas AK, Tzortzis G, Gibson GR (2007b) Molecular cloning and comparative analysis of four β-galactosidase genes from Bifidobacterium bifidum NCIMB41171. Appl Microbiol Biotechnol 76:1365–1372
Hinz SW, van den Broek LA, Beldman G, Vincken JP, Voragen AG (2004) β-Galactosidase from Bifidobacterium adolescentis DSM20083 prefers β(1,4)-galactosides over lactose. Appl Microbiol Biotechnol 66:276–284
Hsu CA, Yu RC, Chou CC (2006) Purification and characterisation of sodium-stimulated β-galactosidase from Bifidobacterium longum CCRC15708. World J Microbiol Biotechnol 22:355–361
Hung MN, Lee BH (2002) Purification and characterisation of a recombinant β-galactosidase with transgalactosylation activity from Bifidobacterium infantis HL96. Appl Microbiol Biotechnol 58:439–445
Katayama T, Sakuma A, Kimura T, Makimura Y, Hiratake J, Sakata K, Yamanoi T, Kumagai H, Yamamoto K (2004) Molecular cloning and characterization of Bifidobacterium bifidum 1,2-α-L-fucosidase (AfcA), a novel inverting glycosidase (glycoside hydrolase family 95). J Bacteriol 186:4885–4893
Kunz C, Rudloff S, Baier W, Klein N, Strobel S (2000) Oligosaccharides in human milk: Structure, functional, and metabolic aspects. Annu Rev Nutr 20:699–722
Mahoney RR (1998) Galactosyl-oligosaccharide formation during lactose hydrolysis: a review. Food Chem 63:147–154
Modler HW (1994) Bifidogenic factors—sources, metabolism and applications. Int Dairy J 4:383–407
Møller PL, Jørgensen F, Hansen OC, Madsen SM, Stougaard P (2001) Intra- and extracellular β-galactosidases from Bifidobacterium bifidum and B. infantis: molecular cloning, heterologous expression, and comparative characterisation. Appl Environ Microbiol 67:2276–2283
Park MS, Yoon HJ, Rhim SL, Ji GE (2001) Molecular cloning and characterisation of the β-galactosidase gene from Bifidobacterium adolescentis Int57. J Microbiol Biotechnol 11:106–111
Podolsky DK (1985) Oligosaccharide structure of human colonic mucin. J Biol Chem 260:8262–8271
Pomeranz Y (1964) Lactase (β-galactosidase). 1. Occurrence and properties. Food Technol 18:682–687
Sambrook J, Russell WD (2001a) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York, USA
Sambrook J, Russell WD (2001b) Expression of cloned genes in Escherichia coli. In: Sambrook J, Russell WD (eds) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York, USA, pp 15.1–15.65
Shipkowski S, Brenchley JE (2006) Bioinformatic, genetic and biochemical evidence that some glycoside hydrolase family 42 β-galactosidases are arabinogalactan type I oligomer hydrolases. Appl Environ Microbiol 72:7730–7738
Tochikura T, Sakai K, Fujiyoshi T, Tachiki T, Kumagai H (1986) p-Nitrophenyl glycoside-hydrolysing activities in Bifidobacteria and characterisation of β-d-galactosidase of Bifidobacterium longum 401. Agric Biol Chem 50:2279–2286
Tzortzis G, Goulas AK, Gibson GR (2005) Synthesis of prebiotic galactooligosaccharides using the whole cells of a novel strain Bifidobacterium bifidum NCIMB41171. Appl Microbiol Biotechnol 68:412–416
van den Broek LA, Hinz SW, Beldman G, Vincken JP, Voragen AG (2008) Bifidobacterium carbohydrases—their role in breakdown and synthesis of (potential) prebiotics. Mol Nutr Food Res 52:146–163
van Laere KM, Abee T, Schols HA, Beldman G, Voragen AG (2000) Characterisation of a novel β-galactosidase from Bifidobacterium adolescentis DSM20083 active towards transgalactooligosaccharides. Appl Environ Microbiol 66:1379–1384
Vetere A, Paoletti S (1998) Separation and characterization of three β-galactosidases from Bacillus circulans. Biochim Biophys Acta 1380:223–231
Wallenfels K, Malhotra OP (1961) Galactosidases. Adv Carbohydr Chem 16:239–298
Whitaker JR (1994) Enzyme cofactors. In: Whitaker JR (ed) Principles of enzymology for the food sciences, 2nd edn. Marcel Dekker, New York, USA, pp 329–365
Acknowledgements
This work was supported by the Greek State Scholarship’s Foundation (IKY).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goulas, T., Goulas, A., Tzortzis, G. et al. Comparative analysis of four β-galactosidases from Bifidobacterium bifidum NCIMB41171: purification and biochemical characterisation. Appl Microbiol Biotechnol 82, 1079–1088 (2009). https://doi.org/10.1007/s00253-008-1795-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1795-5