Abstract
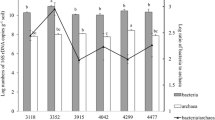
The community ecology, abundance, and diversity patterns of soil archaea are poorly understood—despite the fact that they are a major branch of life that is ubiquitous and important in nitrogen cycling in terrestrial ecosystems. We set out to investigate the elevational patterns of archaeal ecology, and how these compare with other groups of organisms. Many studies of different groups of organisms (plants, birds, etc.) have shown a series of distinct communities with elevation, and often a diversity maximum in mid-elevations. We investigated the soil archaeal communities on Mt. Norikura, Japan, using 454 pyrosequencing of the 16S ribosomal RNA (rRNA) gene. There was a strong mid-elevation maximum in diversity, and a mid-elevation maximum in abundance of soil archaea 16S rRNA and amoA genes. These diversity and abundance maximums could not be correlated with any identifiable soil parameter, nor plant diversity. Discrete, predictable communities of archaea occurred at each elevational level, also not explicable in terms of pH or major nutrients. When we compared the archaeal community and diversity patterns with those found in an earlier study of Mt Fuji, both mountains showed mid-elevation maximums in diversity and abundance of archaea, possibly a result of some common environmental factor such as soil disturbance frequency. However, they showed distinct sets of archaeal communities at similar elevational sampling points. Presumably, the difference reflects their distinct geology (Norikura being andesitic, while Fuji is basaltic) and the resulting combinations of soil chemistry and environmental conditions, although no explanatory variable was found. Clearly, many soil archaea have strongly defined niches and will only occur in a narrow subset of the range of possible climate and soil conditions. The findings of a mid-elevation diversity maximum on Norikura provides a further instance of how widespread this unexplained pattern is in nature, in a wide variety of groups of organisms.







Similar content being viewed by others
References
Woese CR, Kandler O, Wheelis ML (1990) Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A 87:4576–4579
Bintrim SB, Donohue TJ, Handelsman J, Roberts GP, Goodman RM (1997) Molecular phylogeny of archaea from soil. Proc Natl Acad Sci U S A 94:277–282
Buckley DH, Graber JR, Schmidt TM (1998) Phylogenetic analysis of nonthermophilic members of the kingdom Crenarchaeota and their diversity and abundance in soils. Appl Environ Microbiol 64:4333–4339
Oline DK, Schmidt SK, Grant MC (2006) Biogeography and landscape-scale diversity of the dominant crenarchaeota of soil. Microb Ecol 52:480–490. doi:10.1007/s00248-006-9101-5
Ochsenreiter T, Selezi D, Quaiser A, Bonch-Osmolovskaya L, Schleper C (2003) Diversity and abundance of Crenarchaeota in terrestrial habitats studied by 16S RNA surveys and real time PCR. Environ Microbiol 5:787–797. doi:10.1046/j.1462-2920.2003.00476.x
Khelaifia S, Raoult D, Drancourt M (2013) A Versatile Medium for Cultivating Methanogenic Archaea. Plos One 8. doi: ARTN e61563 DOI 10.1371/journal.pone.0061563
Wolfe RS, Metcalf WW (2010) A vacuum-vortex technique for preparation of anoxic solutions or liquid culture media in small volumes for cultivating methanogens or other strict anaerobes. Anaerobe 16:216–219. doi:10.1016/j.anaerobe.2009.11.005
Prosser JI, Nicol GW (2008) Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environ Microbiol 10:2931–2941
Offre P, Spang A, Schleper C (2013) Archaea in biogeochemical cycles. Annu Rev Microbiol 67:437–457. doi:10.1146/annurev-micro-092412-155614
Karner MB, DeLong EF, Karl DM (2001) Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409:507–510. doi:10.1038/35054051
Bates ST, Berg-Lyons D, Caporaso JG, Walters WA, Knight R, Fierer N (2011) Examining the global distribution of dominant archaeal populations in soil. ISME J 5:908–917. doi:10.1038/ismej.2010.171
Lipp JS, Morono Y, Inagaki F, Hinrichs KU (2008) Significant contribution of Archaea to extant biomass in marine subsurface sediments. Nature 454:991–994. doi:10.1038/nature07174
Thauer RK (2011) Anaerobic oxidation of methane with sulfate: on the reversibility of the reactions that are catalyzed by enzymes also involved in methanogenesis from CO2. Curr Opin Microbiol 14:292–299. doi:10.1016/j.mib.2011.03.003
Thauer RK, Kaster AK, Seedorf H, Buckel W, Hedderich R (2008) Methanogenic archaea: ecologically relevant differences in energy conservation. Nat Rev Microbiol 6:579–591. doi:10.1038/nrmicro1931
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809. doi:10.1038/Nature04983
Erguder TH, Boon N, Wittebolle L, Marzorati M, Verstraete W (2009) Environmental factors shaping the ecological niches of ammonia-oxidizing archaea. FEMS Microbiol Rev 33:855–869. doi:10.1111/j.1574-6976.2009.00179.x
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci U S A 102:14683–14688
Bryant JA, Lamanna C, Morlon H, Kerkhoff AJ, Enquist BJ, Green JL (2008) Microbes on mountainsides: contrasting elevational patterns of bacterial and plant diversity. Proc Natl Acad Sci U S A 105:11505–11511. doi:10.1073/pnas.0801920105
Wang JJ, Soininen J, He JZ, Shen J (2012) Phylogenetic clustering increases with elevation for microbes. Environ Microbiol Rep 4:217–226. doi:10.1111/j.1758-2229.2011.00324.x
Shen C, Xiong J, Zhang H, Feng Y, Lin X, Li X, Linag W, Chu H (2012) Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol Biochem. doi:10.1016/j.soilbio.2012.07.013
Singh D, Lee-Cruz L, Kim WS, Kerfahi D, Chun JH, Adams JM (2014) Strong elevational trends in soil bacterial community composition on Mt. Ha lla, South Korea. Soil Biol Biochem 68:140–149
Yang YF, Gao Y, Wang SP, Xu DP, Yu H, Wu LW, Lin QY, Hu YG, Li XZ, He ZL, Deng Y, Zhou JZ (2014) The microbial gene diversity along an elevation gradient of the Tibetan grassland. ISME J 8:430–440. doi:10.1038/ismej.2013.146
Singh D, Takahashi K, Kim M, Chun J, Adams JM (2012) A hump-backed trend in bacterial diversity with elevation on Mount Fuji, Japan. Microb Ecol 63:429–437. doi:10.1007/s00248-011-9900-1
Singh D, Takahashi K, Adams JM (2012a) Elevational patterns in archaeal diversity on Mt. Fuji. Plos One 7. doi: ARTN e44494 DOI 10.1371/journal.pone.0044494
Huston MA (1994) Biological diversity: the coexistence of species on changing landscapes. Cambridge University Press, Cambridge
Verhamme DT, Prosser JI, Nicol GW (2011) Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J 5:1067–1071
Zhang LM, Wang M, Prosser JI, Zheng YM, He JZ (2009) Altitude ammonia-oxidizing bacteria and archaea in soils of Mount Everest. FEMS Microbiol Ecol 70:208–217. doi:10.1111/j.1574-6941.2009.00775.x
Tourna M, Stieglmeier M, Spang A, Konneke M, Schintlmeister A, Urich T, Engel M, Schloter M, Wagner M, Richter A, Schleper C (2011) Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci U S A 108:8420–8425. doi:10.1073/pnas.1013488108
Kim JG, Jung MY, Park SJ, Rijpstra WIC, Sinninghe Damste JS, Madsen EL, Min D, Kim JS, Kim GJ, Rhee SK (2012) Cultivation of a highly enriched ammonia-oxidizing archaeon of thaumarchaeotal group I.1b from an agricultural soil. Environ Microbiol 14:1528–1543
Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P (2008) Mesophilic crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nat Rev Microbiol 6:245–252. doi:10.1038/Nrmicro1852
Sanders NJ, Rahbek C (2012) The patterns and causes of elevational diversity gradients. Ecography 35:1–3. doi:10.1111/j.1600-0587.2011.07338.x
Colwell RK, Hurtt GC (1994) Nonbiological gradients in species richness and a spurious rapoport effect. Am Nat 144:570–595. doi:10.1086/285695
Colwell RK, Lees DC (2000) The mid-domain effect: geometric constraints on the geography of species richness. Trends Ecol Evol 15:70–76. doi:10.1016/S0169-5347(99)01767-X
Wilson JB (1990) Mechanisms of species coexistence—12 explanations for Hutchinson paradox of the plankton - evidence from New Zealand plant-communities. N Z J Ecol 13:17–42
Rahbek C (1995) The elevational gradient of species richness: a uniform pattern? Ecography 18:200–205
Grytnes JA (2003) Species-richness patterns of vascular plants along seven altitudinal transects in Norway. Ecography 26:291–300. doi:10.1034/j.1600-0587.2003.03358.x
Wang XP, Fang JY, Sanders NJ, White PS, Tang ZY (2009) Relative importance of climate vs local factors in shaping the regional patterns of forest plant richness across northeast China. Ecography 32:133–142. doi:10.1111/j.1600-0587.2008.05507.x
Rahbek C (2005) The role of spatial scale and the perception of large-scale species-richness patterns. Ecol Lett 8:224–239. doi:10.1111/j.1461-0248.2004.00701.x
McCain CM (2009) Global analysis of bird elevational diversity. Glob Ecol Biogeogr 18:346–360. doi:10.1111/j.1466-8238.2008.00443.x
Fierer N, McCain CM, Meir P, Zimmermann M, Rapp JM, Silaman MR, Knight R (2011) Microbes do not follow the elevational diversity patterns of plants and animals. Ecology 92:797–804. doi:10.1890/10-1170.1
Takahashi K, Azuma H, Yasue K (2003) Effects of climate on the radial growth of tree species in the upper and lower distribution limits of an altitudinal ecotone on Mount Norikura, central Japan. Ecol Res 18:549–558
Huzimura I (1971) The climate and weather of Mt. Fuji. In: Tsuya, H (ed.) Rep of the Scientific Survey of Mt Fuji, Tokyo, pp. 211–345
Nakano S, Fukuoka T, Aramaki S (1987) Trace-element abundances in the quaternary volcanic-rocks of the Norikura Volcanic Chain, Central Honshu, Japan. Geochem J 21:159–172
Tsuya H (1968) Geology of volcano Mt. Fuji. Explanatory text of geological map 1:50,000 scale, Geological Survey of Japan
Nakano S, Otsuka T, Adachi M, Harayama S, Yoshioka T (1995) Geology of the Norikuradake district, quadrangle series. Kanazawa 10, Geol. Surv. Japan (in Japanese with English abstract)
Ohsawa M (1984) Differentiation of vegetation zones and species strategies in the subalpine region of Mt Fuji. Vegetation 57:15–52
Miyajima Y, Takahashi K (2007) Changes with altitude of the stand structure of temperate forests on Mount Norikura, central Japan. J For Res-Jpn 12:187–192
Miyajima Y, Sato T, Takahashi K (2007) Altitudinal changes in vegetation of tree, herb and fern species on Mount Norikura, central Japan. J Veg Sci 24:29–40
Cadillo-Quiroz H, Brauer S, Yashiro E, Sun C, Yavitt J, Zinder S (2006) Vertical profiles of methanogenesis and methanogens in two contrasting acidic peatlands in central New York State, USA. Environ Microbiol 8:1428–1440
Hur M, Kim Y, Song HR, Kim JM, Choi YI, Yi H (2011) Effect of genetically modified poplars on soil microbial communities during the phytoremediation of waste mine tailings. Appl Environ Microbiol 77:7611–7619. doi:10.1128/AEM.06102-11
Schloss PD, Gevers D, Westcott SL (2011) Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS ONE 6
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721. doi:10.1099/ijs.0.038075-0
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. doi:10.1093/bioinformatics/btr381
Hill TCJ, Walsh KA, Harris JA, Moffett BF (2003) Using ecological diversity measures with bacterial communities. FEMS Microbiol Ecol 43:1–11. doi:10.1016/S0168-6496(02)00449-X
Faith DP (1992) Conservation evaluation and phylogenetic diversity. Biol Conserv 61:1–10
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) Vegan: community ecology package. R package version 2.0-8
Sarle WS (1990) The VARCLUS procedure. SAS/STAT User’s Guide. SAS Institute, Inc., Cary NC
Legendre P, Lapointe FJ, Casgrain P (1994) Modeling brain evolution from behavior: a permutational regression approach. Evolution 48:1487–1499
Clarke KR, Gorley RN (2006) Primer v6: user manual/tutorials. Primer-E Ltd, Plymouth, UK
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci U S A 103:626–631. doi:10.1073/pnas.0507535103
Rousk J, Baath E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–1351. doi:10.1038/ismej.2010.58
Kubo K, Lloyd KG, Biddle JF, Amann R, Teske A, Knittel K (2012) Archaea of the miscellaneous crenarchaeotal group are abundant, diverse and widespread in marine sediments. ISME J 6:1949–1965
Timonen S, Bomberg M (2009) Archaea in dry soil environments. Phytochem Rev 8:505–518
Lehtovirta LE, Prosser JI, Nicol GW (2009) Soil pH regulates the abundance and diversity of Group 1.1c Crenarchaeota. FEMS Microbiol Ecol 70:367–376. doi:10.1111/j.1574-6941.2009.00748.x
Bomberg M, Timonen S (2009) Effect of tree species and mycorrhizal colonization on the archaeal population of Boreal Forest Rhizospheres. Appl Environ Microbiol 75:308–315
Weber EB, Lehtovirta LE, Prosser JI, Gubry-Rangin C (2015) Ammonia oxidation is not required for growth of Group 1.1c soil Thaumarchaeota. FEMS Microbiol Ecol 91. doi: http://dx.doi.org/10.1093/femsec/fiv001
Kemnitz D, Kolb S, Conrad R (2007) High abundance of Crenarchaeota in a temperate acidic forest soil. FEMS Microbiol Ecol 60:442–448
Margesin R, Jud M, Tscherko D, Schinner F (2009) Microbial communities and activities in alpine and subalpine soils. FEMS Microbiol Ecol 67:208–218. doi:10.1111/j.1574-6941.2008.00620.x
Sundqvist MK, Liu Z, Giesler R, Wardle DA (2013) Plant and microbial responses to nitrogen and phosphorus addition across an elevational gradient in subarctic tundra. Ecology. doi:10.1890/13-0869.1
Korner C (2007) The use of ‘altitude’ in ecological research. Trends Ecol Evol 22:569–574. doi:10.1016/j.tree.2007.09.006
Frijlink MJ, Abee T, Laanbroek HJ, Deboer W, Konings WN (1992) The bioenergetics of ammonia and hydroxylamine oxidation in nitrosomonas-europaea at acid and alkaline Ph. Arch Microbiol 157:194–199
Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA (2009) Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461:976–U234
Park HD, Noguera DR (2007) Characterization of two ammonia-oxidizing bacteria isolated from reactors operated with low dissolved oxygen concentrations. J Appl Microbiol 102:1401–1417
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2010) Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol Ecol 72:386–394. doi:10.1111/j.1574-6941.2010.00861.x
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
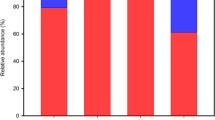
Archaeal phyla breakdown for Mt. Fuji (a) and Mt. Norikura (b) (GIF 35 kb)
Supplementary Fig. S2
Relative abundance of the four most abundant archaeal sub-phyla on Mt. Fuji (a) and Mt. Norikura (b) (Mean ± SE) in relation to pH. Significance level is shown at ***P < 0.001, **P < 0.01, and *P < 0.05. (GIF 38 kb)
Supplementary Fig. S3
Distribution of Thaumarchaeal group I.1b percent relative abundance (solid line; pyrosequencing results) vs. amoA gene copies per gram of soil (dashed line; qPCR results) in relation to elevation on Mt. Fuji (a) and Mt. Norikura (b). Data from biological replicates at each elevational zones were averaged prior to statistical analysis. (GIF 17 kb)
Supplementary Fig. S4
NMDS plot on the Euclidean distances from normalized environmental variables. (GIF 14 kb)
Supplementary Fig. S5
Venn diagram representing overall overlap of OTUs for Thaumarchaeal Group I.1b (GIF 44 kb)
ESM 1
(XLSX 20 kb)
ESM 2
(XLSX 17 kb)
ESM 3
(XLSX 13 kb)
Rights and permissions
About this article
Cite this article
Singh, D., Takahashi, K., Park, J. et al. Similarities and Contrasts in the Archaeal Community of Two Japanese Mountains: Mt. Norikura Compared to Mt. Fuji. Microb Ecol 71, 428–441 (2016). https://doi.org/10.1007/s00248-015-0681-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-015-0681-9