Abstract
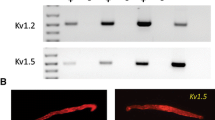
Strong inwardly rectifying K+ (KIR) channels that contribute to maintaining the resting membrane potential are encoded by the Kir2.0 family (Kir2.1–2.4). In smooth muscle, KIR currents reported so far have the characteristics of Kir2.1. However, Kir2.4, which exhibits unique characteristics of barium block, has been largely overlooked. Using patch-clamp techniques, we characterized KIR channels in cultured human pulmonary artery smooth muscle (HPASM) cells and compared them to cloned Kir2.1 and Kir2.4 channels. In a physiological K+ gradient, inwardly rectifying currents were observed in HPASM cells, the magnitude and reversal potential of which were sensitive to extracellular K+ concentration. Ba2+ (100 μM) significantly inhibited inward currents and depolarized HPASM cells by ∼10 mV. In 60 mM extracellular K+, Ba2+ blocked KIR currents in HPASM cells with a 50% inhibitory concentration of 39.1 μM at –100 mV compared to 3.9 μM and 65.6 μM for Kir2.1 and Kir2.4, respectively. Cloned Kir2.4 and KIR currents in HPASM cells showed little voltage dependence to Ba2+ inhibition, which blocked at a more superficial site than for Kir2.1. Single-channel recordings revealed strong inwardly rectifying channels with an average conductance of 21 pS in HPASM cells, not significantly different from either Kir2.1 (19.6 pS) or Kir2.4 (19.4 pS). Reverse-transcription polymerase chain reaction detected products corresponding to Kir2.1, Kir2.2 and Kir2.4 but not Kir2.3. We demonstrate that cultured HPASM cells express KIR channels and suggest both Kir2.1 and Kir2.4 subunits contribute to these channels, although the whole-cell current characteristics described share more similarity with Kir2.4.






Similar content being viewed by others
References
Alioua A., Conti L., Eghbali M., Mahajan A., Tanaka Y., Stefani E., Vandenberg C., Toro L. 2003. Inward rectifier K+ channels (Kir) control muscle tone of a rat conduit vessel: Role of Kir2.x. Biophys. J. 84:225A
Bradley K.K., Jaggar J.H., Bonev A.D., Heppner T.J., Flynn E.R.M., Nelson M.T., Horowitz B. 1999. Kir2.1 encodes the inward rectifier potassium channel in rat arterial smooth muscle. J. Physiol. 515:639–651
Cui Y., Giblin J.P., Clapp L.H., Tinker A. 2001. A mechanism for ATP-sensitive potassium channel diversity: Functional coassembly of two pore forming subunits. Proc. Natl. Acad. Sci. USA 98:729–734
Cui Y., Tran S., Tinker A., Clapp L.H. 2002. The molecular composition of KATP channels in human pulmonary artery smooth muscle cells and their modulation by growth. Am. J. Respir. Cell. Mol. Biol. 26:135–143
Edwards F.R., Hirst G.D.S., Silverberg G.D. 1988. Inward rectification in rat cerebral arterioles: Involvement of potassium ions in autoregulation. J. Physiol. 404:455–466
Edwards G., Weston A.H. 2004. Potassium and potassium clouds in endothelium-dependent hyperpolarizations. Pharmacol. Res. 49:535–541
Fang Y., Schram G., Romanenko V.G., Shi C., Conti L., Vandenberg C.A., Davies P.F., Nattel S., Levitan I. 2005. Functional expression of Kir2.x in human aortic endothelial cells: The dominant role of Kir2.2. Am. J. Physiol. 289:C1134–C1144
Flynn E.R.M., McManus C.A., Bradley K.K., Koh S.D., Hegarty T.M., Horowitz B., Sanders K.M. 1999. Inward rectifier potassium conductance regulates membrane potential of canine smooth muscle. J. Physiol. 518:247–256
Giblin J.P., Leaney J.L., Tinker A. 1999. The molecular assembly of ATP-sensitive potassium channels: Determinants on the pore forming subunit. J. Biol. Chem. 274:22652–22659
Hoger J.H., Ilyin V.I., Forsyth S., Hoger A. 2002. Shear stress regulates the endothelial Kir2.1 ion channel. Proc. Natl. Acad. Sci. USA 99:7780–7785
Hogg D.S., McMurray G., Kozlowski R.Z. 2002. Endothelial cells freshly isolated from small pulmonary arteries of the rat possess multiple distinct K+ current profiles. Lung 180:203–214
Hughes B.A., Kumar G., Yuan Y., Swaminathan A., Yan D., Sharma A., Plumley L., Yang-Feng T.L., Swaroop A. 2000. Cloning and functional expression of human retinal Kir2.4, a pH-sensitive inwardly rectifying K+ channel. Am. J. Physiol. 279:C771–C784
Kamouchi M., Van Den Bremt K., Eggermont J., Droogmans G., Nilius B. 1997. Modulation of inwardly rectifying potassium channels in cultured bovine pulmonary artery endothelial cells. J. Physiol. 504:545–556
Knot H.J., Zimmermann P.A., Nelson M.T. 1996. External K+ induced dilations of rat coronary and cerebral arteries involve inward rectifier K+ channels. J. Physiol. 492:419–430
Liu G.X., Derst C., Schlichthorl G., Heinen S., Seebohm G., Bruggemann A., Kummer W., Veh R.W., Daut J., Preisig-Muller R. 2001. Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes. J. Physiol. 532:115–126
Michelakis E.D., Weir E.K., Wu X., Nsair A., Waite R., Hashimoto K., Puttagunta L., Knaus H.G., Archer S.L. 2001. Potassium channels regulate tone in rat pulmonary veins. Am. J. Physiol. 280:L1138–L1147
Nakamura T.Y., Lee K., Artman M., Rudy B., Coetzee W.A. 1999. The role of Kir2.1 in the genesis of native cardiac inward-rectifier K+ currents during pre- and postnatal development. Ann. N. Y. Acad. Sci. 868:434–437
Nichols C.G., Lopatin A.N. 1997. Inward rectifier potassium channels. Annu. Rev. Physiol. 59:171–191
Nilius B. Droogmans G. 2001. Ion channels in the vascular endothelium. Physiol. Rev. 81:1415–1459
Oonuma H., Iwasawa K., Iida H., Nagata T., Imuta H., Morita Y., Yamamoto K., Nagai R., Omata M., Nakajima T. 2002. Inward rectifier K+ current in human bronchial smooth muscle cells: Inhibition with antisense oligonucleotides targeted to Kir2.1 mRNA. Am. J. Respir. Cell Mol. Biol. 26:371–379
Orie N.N., Fry C.H., Clapp L.H. 2006. Evidence that inward rectifier K+ channels mediate relaxation by the PGI2 receptor agonist cicaprost via a cyclic AMP-independent mechanism. Cardiovasc. Res. 69:107–115
Preisig-Muller R., Schlichthorl G., Goerge T., Heinen S., Bruggemann A., Rajan S., Derst C., Veh R.W., Daut J. 2002. Heteromerization of Kir2.x potassium channels contributes to the phenotype of the Andersen’s syndrome. Proc. Natl. Acad. Sci. USA 99:7774–7779
Quayle J.M., Dart C., Standen N.B. 1996. The properties and distribution of inward rectifier potassium currents in pig coronary arterial smooth muscle. J. Physiol. 494:715–726
Quayle J.M., Mccarron J.G., Brayden J.E., Nelson M.T. 1993. Inward rectifier K+ currents in smooth muscle cells from rat resistance-sized cerebral arteries. Am. J. Physiol. 265:C1363–C1370
Quayle J.M., Nelson M.T., Standen N.B. 1997. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol. Rev. 77:1166–1232
Robertson B.E., Bonev A.D., Nelson M.T. 1996. Inward rectifier K+ currents in smooth muscle cells from rat coronary arteries: Block by Mg2+, Ca2+, and Ba2+. Am. J. Physiol. 40:H696–H705
Sakai H., Shimizu T., Hori K., Ikari A., Asano S., Takeguchi N. 2002. Molecular and pharmacological properties of inwardly rectifying K+ channels of human lung cancer cells. Eur. J. Pharmacol. 435:125–133
Schram G., Melnyk P., Pourrier M., Wang Z., Nattel S. 2002. Kir2.4 and Kir2.1 K+ channel subunits co-assemble: A potential new contributor to inward rectifier current heterogeneity. J. Physiol 544:337–349
Shimoda L.A., Welsh L.E., Pearse D.B. 2002. Inhibition of inwardly rectifying K+ channels by cGMP in pulmonary vascular endothelial cells. Am. J. Physiol. 283:L297–L304
Snetkov V.A., Ward J.P.T. 1999. Ion currents in smooth muscle cells from human small bronchioloes: Presence of an inward rectifier K+ current and three types of large conductance K+ channels. Exp. Physiol. 84:835–846
Stanfield P.R., Nakajima S., Nakajima Y. 2002. Constitutively active and G-protein coupled inward rectifier K+ channels: Kir2.0 and Kir3.0. Rev. Physiol. Biochem. Pharmacol. 145:47–179
Stonehouse A.H., Pringle J.H., Norman R.I., Stanfield P.R., Conley E.C., Brammar W.J. 1999. Characterisation of Kir2.0 proteins in the rat cerebellum and hippocampus by polyclonal antibodies. Histochem. Cell Biol. 112:457–465
Topert C., Doring F., Wischmeyer E., Karschin C., Brockhaus J., Ballanyi K., Derst C., Karschin A. 1998. Kir2.4: A novel K+ inward rectifier channel associated with motoneurons of cranial nerve nuclei. J. Neurosci. 18:4096–4105
Voets T., Droogmans G., Nilius B. 1996. Membrane currents and the resting membrane potential in cultured bovine pulmonary artery endothelial cells. J. Physiol. 497:95–107
Woodhull A.M. 1973. Ionic blockage of sodium channels in nerve. J. Gen. Physiol 61:687–708
Zaritsky J.J., Eckman D.M., Wellman G.C., Nelson M.T., Schwarz T.L. 2000. Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K+ current in K+-mediated vasodilation. Circ. Res. 87:160–166
Zaritsky J.J., Redell J.B., Tempel B.L., Schwarz T.L. 2001. The consequences of disrupting cardiac inwardly rectifying K+ current (IK1) as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes. J. Physiol. 533:697–710
Acknowledgment
This work was supported by the British Heart Foundation (PG 99176, PG/03/062). L. H. C. is a Medical Research Council Senior Fellow in Basic Science (G117/440).
Author information
Authors and Affiliations
Corresponding author
Additional information
The first two authors contributed equally to the work.
Rights and permissions
About this article
Cite this article
Tennant, B.P., Cui, Y., Tinker, A. et al. Functional Expression of Inward Rectifier Potassium Channels in Cultured Human Pulmonary Smooth Muscle Cells: Evidence for a Major Role of Kir2.4 Subunits. J Membrane Biol 213, 19–29 (2006). https://doi.org/10.1007/s00232-006-0037-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-006-0037-y