Abstract
Introduction
Selective decontamination of the digestive tract (SDD) is a strategy in mechanically ventilated patients to reduce mortality. Treatment consists of enterally administered non-absorbable antibiotics, i.e., tobramycin. However, most intensive care unit (ICU) patients with SDD appear to have detectable tobramycin serum concentrations. The Rijnstate Hospital implemented a protocol for therapeutic drug monitoring (TDM) of tobramycin in patients at risk. The aim of this study was to evaluate the necessity of TDM in these patients and to optimize the current protocol.
Methods
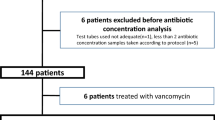
This retrospective observational study included ICU patients with SDD treatment for ≥ 7 days and renal failure. These patients were considered eligible for monitoring of tobramycin. Tobramycin serum concentrations, relevant laboratory parameters (i.e., renal function, lactate), and patient data were extracted from the National Intensive Care Evaluation database and the hospital electronic patient data system.
Results
In 23 subjects, a total of 43 tobramycin serum concentrations was determined. The median tobramycin serum concentration was 0.33 (IQR 0.17–0.49) mg/L of which 12 (27.9%) samples had concentrations < 0.2 mg/L, 30 (69.8%) had concentrations 0.2–1.0 mg/L and 1 (2.3%) had a toxic concentration > 1.0 mg/L. In 3 (7.0%) cases, an intervention was conducted based on the tobramycin serum concentration.
Conclusion
The majority (83.7%) of samples had detectable tobramycin serum concentrations. Monitoring of tobramycin serum concentrations can be considered necessary in patients at risk. However, the current protocol should be optimized to intercept patients more precise.


Similar content being viewed by others
References
Houben A J M AJ (2014) Selective decontamination of the oropharynx and the digestive tract, and antimicrobial resistance: a 4 year ecological study in 38 intensive care units in the Netherlands. J Antimicrob Chemother 69(3):797–804
Oostdijk EA Selective decontamination in ICU patients: Dutch guideline. Hospital 9(3):0–5
Oudemans-van Straaten HMHM (2011) Presence of tobramycin in blood and urine during selective decontamination of the digestive tract in critically ill patients, a prospective cohort study. Crit Care 15(5):R240
Mol Meri M (2008) Systemic tobramycin concentrations during selective decontamination of the digestive tract in intensive care unit patients on continuous venovenous hemofiltration. Intensive Care Med 34(5):903–906
Naber KGKG (1973) Pharmacokinetics of the aminoglycoside antibiotic tobramycin in humans. Antimicrob Agents Chemother 3(4):469–473
Hoste Eric AJEA (2015) Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 41(8):1411–1423
Wargo Kurt AKA (2014) Aminoglycoside-induced nephrotoxicity. J Pharm Pract 27(6):573–577
SWAB-Richtlijn: selectieve decontaminatie bij patiënten op de intensive care. Stichting Werkgroep Antibioticabeleid. Http://www.swab.nl/swab/cms3.nsf/uploads/E6FA609CFB1010C3C1257D4D0031DAD6/$FILE/Richtlijn%20SDD%202014.pdf. Publication August 2014. Revision August 2019. Accessed May 22, 2017
Trotman Robin LRL (2005) Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin Infect Dis 41(8):1159–1166
Pazhayattil George Sunny GS (2014) Drug-induced impairment of renal function. Int J Nephrol Renov Dis 7:457–468
Karaiskos I, Friberg LE, Galani L, Ioannidis K, Katsouda E, Athanassa Z, Paskalis H, Giamarellou H (2016) Challenge for higher colistin dosage in critically ill patients receiving continuous venovenous haemodiafiltration. Int J Antimicrob Agents 48(3):337–341
Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ (2002) Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob Agents Chemother 46(3):834–840
Holte KK (2000) Postoperative ileus: a preventable event. Br J Surg 87(11):1480–1493
Author information
Authors and Affiliations
Contributions
J.E. Möhlmann PharmD. Collected the data, analyzed the results and wrote the paper.
M. van Luin PharmD PhD. Second supervisor. Contributed to paper with text and comments. Co-inventor of the analyzed TDM protocol.
E.M. Mascini MD PhD. Contributed to the paper with text and comments. Co-inventor of the analyzed TDM protocol.
H.J. van Leeuwen MD PhD. Contributed to the paper with text and comments. Co-inventor of the analyzed TDM protocol. Provided guidance during internship at the department of Intensive Care.
M.R. de Maat PharmD PhD. First supervisor. Contributed to the papier with text and comments. Co-inventor of the analyzed TDM protocol.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval for research involving human participants, informed consent
This study was approved by the local medical ethical review board of the Rijnstate Hospital. Requirement of informed consent was waived for this retrospective study since determination of tobramycin serum concentrations was part of routine care in selected patients within the SDD regimen.
Additional information
Data availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 15.5 kb)
Rights and permissions
About this article
Cite this article
Möhlmann, J.E., van Luin, M., Mascini, E.M. et al. Monitoring of tobramycin serum concentrations in selected critically ill patients receiving selective decontamination of the digestive tract: a retrospective evaluation. Eur J Clin Pharmacol 75, 831–836 (2019). https://doi.org/10.1007/s00228-019-02644-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-019-02644-x