Abstract
Purpose
To evaluate the relationship between total and free MPA pharmacokinetic (PK) parameters and renal outcome markers, and to verify whether conducting therapeutic drug monitoring (TDM) in lupus nephritis (LN) patients would be of value in routine clinical practice.
Methods
Eighty-four samples were collected from sixteen LN patients. Total and free MPA concentrations were measured at predose, 0.5 and 2 h after mycophenolate mofetil (MMF) intake. Area under the concentration time curve from 0 to 2 h (AUC0–2) and free fraction were calculated.
Results
High between-patient variability was observed (CV% of 53.5% for dose-normalized total MPA AUC0–2). A significant but weak correlation between dose-normalized total C0 and AUC0–2 was noted (r = 0.5699). Dose-normalized total C0 above 2.76 μg/mL·g may indicate patients with eGFR < 81 mL/min with sensitivity of 83.3% and specificity of 75.0%. Hypoalbuminemic LN patients demonstrated significantly elevated MPA free fraction when compared with patients with serum albumin concentration ≥ 3.5 g/dL (1.49 ± 0.64% vs 1.08 ± 0.75%).
Conclusion
This study examined relationship between free and total pharmacokinetic MPA parameters as well as the effect of hypoalbuminemia on MPA plasma protein binding in adult LN patients. The study results suggest that TDM of MPA in LN seems to be a more reasonable approach than the fixed-dose protocol. Moreover, predose total MPA concentration may be a possible estimation of MPA exposure, while monitoring free rather than total MPA may be more beneficial in hypoalbuminemic patients.
Similar content being viewed by others
Introduction
Mycophenolate mofetil (MMF), a prodrug of an immunosuppressive agent mycophenolic acid (MPA), is recommended by the Joint European League Against Rheumatism (EULAR) and European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) for the management of class III and IV lupus nephritis (LN) in induction as well as the maintenance therapy [1]. MPA pharmacokinetics is characterized by the considerable inter- and intrapatient variability thus fixed dose approach in the LN population may not be beneficial for the renal outcome improvement in every patient [2]. Monitoring of MPA plasma concentrations in transplant patients resulted in the reduction of the acute graft rejection episodes occurrence [3, 4]. To date, there are no recommendations regarding therapeutic drug monitoring (TDM) in LN [1, 5] although it was demonstrated that MPA exposure correlates with the treatment outcome [6, 7]. This suggests that dose adjustment to the MPA target concentrations range in the LN patients may be more beneficial than standard fixed dosing protocol. However, it still needs to be evaluated as limited number of studies have been published so far [5].
Free MPA concentrations monitoring may also be of particular importance for TDM in LN. There are several conditions, such as hypoalbuminemia, in which MPA exposure would not be predicted accurately by the routinely monitored total plasma concentrations [8]. It is due to the fact that in individuals with normal renal and liver outcomes MPA is 97 to 99% bound to albumin. Any alterations in this binding may cause elevated free MPA concentrations with minor or even no effect on total MPA concentrations [8,9,10].
According to our knowledge, there were only two studies published that explored the effect of both total and free MPA exposure on adult LN patients outcome [2, 11]. However, none of them has evaluated the relationship between corresponding free and total MPA pharmacokinetic (PK) parameters and the effect of hypoalbuminemia on MPA plasma protein binding. Taking into account the relevance of free MPA monitoring and the scarce data available, we decided to conduct PK study in adult patients with class III and IV LN being on MMF therapy to evaluate the relationship between total and free MPA PK parameters and renal outcome markers, and to verify whether conducting TDM in LN patients would be of value in routine clinical practice.
Methods
Study design
Patients with LN from the Department of Transplantation Medicine, Nephrology and Internal Medicine of Medical University of Warsaw (Warsaw, Poland) and the Department and Clinic of Nephrology and Transplantation Medicine of Wroclaw Medical University (Wroclaw, Poland) that met inclusion criteria were enrolled in the study. The following inclusion criteria were adhered to: (1) biopsy-proven class III and IV lupus nephritis (LN) (according to the International Society of Nephrology/Renal Pathology Society classification from 2003 [12]) (2) age > 18 years (3) treatment with MMF. Patients who received medicinal products known to be interacting with MPA were excluded from the study. Concurrent treatment with antihypertensive drugs or chloroquine was permitted and recorded. The study was approved by the local ethics committee. Informed consent was obtained from all participants.
Patients included in the study
The detailed patients’ characteristics are given in Table 1. Sixteen biopsy-proven LN patients met the inclusion criteria providing a total of 26 three-time point and 3 two-time point abbreviated 2 h profiles. Former intake of cyclophosphamide was noted for six participants. Concurrent treatment included glucocorticosteroids, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers and chloroquine, and was received by 16, 13, and 4 patients, respectively. MMF doses taken twice a day were distributed as follows: 250 mg (1 profile), 500 mg (7 profiles), 1000 mg (8 profiles), and 1500 mg (1 profile). Additionally, in case of one profile MMF dose of 500 mg was received 3 times a day, while in 1 patient the twice-daily dose was divided into 1000 mg and 500 mg. All of the patients were Caucasians.
Patients excluded from the study
Two patients were excluded from the study due to the intake of cyclosporine known to decrease MPA exposure due to the influence on MPA enterohepatic circulation [13]. Moreover, there was a group of eight patients that could not be included in the study since it was impossible to determine both total and free MPA profiles due to the insufficient plasma volume collected. Although the following results could not be included in the study analysis with no total MPA concentrations, still free MPA concentrations were measured in the samples from those eight patients providing 11 three-time point abbreviated 2 h profiles for future reference.
Analytical methods
Steady-state blood samples were collected at predose and 0.5 and 2 h after the morning dose of MMF by venipuncture using EDTA tubes. All samples were subsequently centrifugated to obtain plasma which was then stored at −20 °C until analysis.
Total MPA plasma concentrations were measured by the validated high-performance liquid chromatography method with ultraviolet detection (HPLC-UV) used in our laboratory since 2003. It is a modification of Shaw et al. [14] method and was detailed previously [15]. Its analytical performance was assured by continuous participation in the Mycophenolate International Proficiency Testing Scheme provided then by Analytical Services International (London, UK).
Free MPA concentrations in plasma ultrafiltrate were determined after prior ultrafiltration with Centrifree Micropartition System® (Merck Millipore, Co. Cork, Ireland) by liquid chromatography–tandem mass spectrometry (LC-MS/MS) method developed, validated, and described in detail recently [16].
Pharmacokinetic analysis
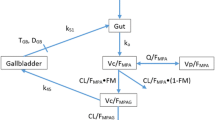
Taking the practical aspects of the organization of patient care into consideration, the samples were collected only until 2 h after MMF intake. Total and free MPA concentrations at three time points (C0, C0.5, C2) were then determined. Review of current literature revealed no limited sampling strategy (LSS) model developed and validated in LN population for the above sample times. Therefore, non-compartmental analysis was performed to calculate the area under the concentration time curve from 0 to 2 h (AUC0–2) by linear trapezoidal rule using WinNonlin 3.2 Pro software (Pharsight Inc., Mountain View, CA, USA). PK parameters were normalized to the actual morning dose of MMF. Free fraction (Ff) was calculated according to the following formula: Ff = Cfree / Ctotal · 100%.
Outcome measurements
The following biochemical parameters were determined at the time of plasma sampling: serum creatinine, estimated glomerular filtration rate (eGFR), serum albumin, urine protein (UP), 24-h urine protein (24UP), hemoglobin level (Hb), and white blood cell count (WBC). Comparisons of PK parameters values were made between patients grouped by the following demographic and biochemical factors: gender, eGFR (< 81 mL/min vs ≥ 81 mL/min), 24UP (≤ 0.5 g/day vs > 0.5 g/day), and serum albumin (< 3.5 g/dL vs ≥ 3.5 g/dL). The cutoff points for the eGFR and 24UP factors were determined based on the European League Against Rheumatism (EULAR) definition of partial and complete renal response [17]. According to the EULAR criteria, the value of eGFR above 81 mL/min and the value of 24UP below 0.5 g/day indicate partial and complete response to the immunosuppressive treatment. Serum albumin level below 3.5 g/dL was chosen as an indicator of hypoalbuminemia [8]. The comparison between patient groups with and without decreased albumin concentration is particularly valuable due to its significant influence on free MPA plasma concentrations [8,9,10].
Statistical analyses
All statistical analyses, as well as box-plots and scatterplots, were performed and created using Dell Statistica software, version 13 (Dell Inc., Tulsa, OK, USA). p value < 0.05 was considered significant. Quantitative data were presented as mean ± SD and median (range), while qualitative data as numbers and frequencies (percentage). Nonparametric Mann-Whitney U test was used to evaluate the differences in PK parameters between patients grouped by demographic and biochemical factors (see “Outcome measurements”), while Kruskal-Wallis ANOVA test was used to evaluate the differences in MPA free fraction between three sampling time points. To meet the independence assumption of Mann-Whitney U and Kruskal-Wallis ANOVA tests, only 16 profiles (one profile provided for a given patient) were considered for the above-mentioned statistical analyses. Spearman rank correlation was used to establish the relationship between PK parameters and biochemical data. Receiver operating characteristic (ROC) curves (sensitivity versus 1-specificity) of PK parameters were analyzed to discriminate between LN patients grouped by the biochemical factors (see “Outcome measurements”). The area under the ROC curve was estimated according to the Hanley and McNeil method [18].
Results
Pharmacokinetics
Total MPA, free MPA, and MPA free fraction results are presented in Table 2, while Supplementary Fig. 1 shows its abbreviated 2 h profiles. The mean maximum concentration in case of both total and free MPA was observed 0.5 h after MMF intake. An important result was that only 9 out of 28 samples (32.1%) for total MPA predose concentration exceeded the border value of 3 μg/mL recommended in the literature for LN treatment [5, 19]. No statistically significant differences for MPA free fraction between three time points (p = 0.7547) were observed. MPA free fraction ranged between 0.31–3.43% and yielded 1.08 ± 0.65%. A total of 50 out of 84 measurements (59.5%) demonstrated free fraction lower than 1%.
Results of free MPA concentrations measured in the samples in which plasma volume was insufficient to determine also total MPA concentrations (see “Patients excluded from the study”) are presented in Supplementary Table 1.
Pharmacokinetic comparisons between groups
Among all of the PK parameters compared between patients grouped by gender MPA free fraction measured 2 h after MMF intake was the only one that differed significantly (p = 0.0250).
The comparisons regarding two biochemical factors related to therapeutic response, 24UP and eGFR, were made as well. In patients grouped by 24UP value total MPA predose concentrations were significantly higher (p = 0.0311) in LN patients with 24UP value > 0.5 g/day (2.84 ± 1.42 μg/mL) than in patients with 24UP value (≤ 0.5 g/day) (1.50 ± 1.16 μg/mL). In case of eGFR factor, both dose-normalized total and free MPA predose concentrations were significantly higher (p = 0.0033 and p = 0.0164, respectively) in LN patients with eGFR value < 81 mL/min than in patients with eGFR ≥ 81 mL/min. Dose-normalized total MPA C0 amounted to 4.59 ± 2.32 μg/mL·g vs 1.41 ± 1.15 μg/mL·g, while dose-normalized free MPA C0 amounted to 43.50 ± 31.40 ng/mL·g vs 17.34 ± 10.83 ng/mL·g (see Supplementary Fig. 2).
Additionally, PK parameters were compared depending on serum albumin concentration. It turned out that hypoalbuminemic LN patients (serum albumin below 3.5 g/dL) demonstrated significantly (p = 0.0276) elevated MPA free fraction when compared with patients with serum albumin concentration ≥ 3.5 g/dL (1.49 ± 0.64% vs 1.08 ± 0.75%) (see Supplementary Fig. 3).
The abovementioned analyses were made on 16 profiles following the independence assumptions of statistical tests used (see “Statistical analyses”). Nevertheless, although statistically not fully correct, it is worth mentioning that inference made on the whole number of 29 profiles using nonparametric approach led to very similar conclusions regarding patients grouped by gender, eGFR, and serum albumin concentration.
Correlation of pharmacokinetic parameters with biochemical data
Several relevant correlations were observed between PK parameters and biochemical markers.
Dose-normalized total C0 significantly correlated with eGFR (r = −0.3808, p = 0.0456, n = 28), while neither PK parameter was related significantly with 24UP.
Regarding MPA plasma protein binding it was observed that MPA free fraction increased with serum albumin concentration decrease (see Fig. 1).
Relationship between pharmacokinetic parameters
PK parameters were subject to evaluate correlation with one another. Some important findings were made and presented in Supplementary Table 2, while crucial correlations are demonstrated in Fig. 2. Namely, total and free C0 were significantly correlated with total AUC0–2. Moreover, valid relationship was noted between free C0 and free AUC0–2. Furthermore, relationships between corresponding total and free MPA PK parameters (C0, AUC0–2) were statistically significant. MPA free fraction, however, was significantly related only with free but not total AUC0–2.
Correlation between mycophenolic acid pharmacokinetic parameters. The relationship between dose-normalized (A) total AUC0–2 and total C0, (B) free AUC0–2 and free C0, (C) free and total AUC0–2, (D) free and total C0 was significant with p value < 0.05. 95% confidence interval is marked with dashed lines
Also, it was found that there is a statistically evident positive correlation between MMF daily dose and total C2 (r = 0.5803, p = 0.0010, n = 29), free C2 (r = 0.4727, p = 0.0096, n = 29), and total AUC0–2 (r = 0.5083, p = 0.0080, n = 26).
Receiver operating characteristic curves analysis
Taking into account findings described in “Pharmacokinetic comparisons between groups” and “Correlation of pharmacokinetic parameters with biochemical data,” the ROC curves analysis was undertaken to verify whether there is a specific value of a given PK parameter that could discriminate between LN patients with eGFR below and above 81 mL/min, with 24UP below and above 0.5 g/day and between patients with and without hypoalbuminemia (serum albumin < 3.5 g/dL).
Regarding eGFR parameter, there were two significant classification models obtained (p < 0.05) (see Fig. 3). According to ROC curve analysis, dose-normalized total C0 above 2.76 μg/mL·g may distinguish patients with eGFR < 81 mL/min with a diagnostic sensitivity of 83.3% and diagnostic specificity of 75.0%. An auspicious classification value of this model is highlighted by ROC-AUC of 0.802. The other ROC curve model, however, analyzing dose-normalized free C0 was characterized by a lower ROC-AUC of 0.755. Nevertheless, its value above 43.40 ng/mL·g may indicate patients with eGFR value below 81 mL/min with a diagnostic sensitivity of 50.0% and diagnostic specificity of 88.2%.
Significant classification models (p < 0.05) obtained by receiver operating characteristic curves analysis (A) to discriminate between lupus nephritis patients with eGFR values below and above 81 mL/min, (B) to discriminate between patients with and without hypoalbuminemia (serum albumin < 3.5 g/dL). The reference line indicates no discrimination between groups. Proposed cutoff and ROC-AUC values are presented
In accordance with the results demonstrated above, also ROC curve analysis did not provide any significant classification models that could distinguish patients with 24UP value > 0.5 g/day.
Contrary, there were two significant classification models obtained to discriminate between LN patients with and without hypoalbuminemia (< 3.5 g/dL) (see Fig. 3). According to ROC curve analysis, free AUC0–2 above 295.2 ng·h/mL and free fraction above 1.85% may indicate hypoalbuminemic patients with diagnostic sensitivity of 40.0% and 35.7%, respectively and diagnostic specificity of 93.3% and 89.4%, respectively. ROC-AUC for both models was 0.760 and 0.736, respectively.
Discussion
According to our knowledge, there have been two studies so far which evaluated not only total but also free MPA pharmacokinetics in adult LN patients [2, 11]. However, our report presents research that additionally examined relationship between free and total MPA concentration-related parameters in this particular population of patients. We also demonstrated the effect of hypoalbuminemia on MPA plasma protein binding in LN patients. The results of these unique analyses may bring some helpful information when formulating in the future TDM recommendations in LN.
It has been concluded based on literature review that total MPA predose concentration value of 3 μg/mL may be associated with a higher probability of LN remission [1, 5]. In our study, only 32.1% of samples reached the recommended value; however, it should be beared in mind that standard MMF fixed-dosing protocol was administered in both clinical centers. Moreover, high between-patient variability in MPA-shortened AUC was observed with extremes of 5.69 and 50.05 μg·h/mL·g (CV% of 53.5%) as well as 37.97 and 590.5 ng·h/mL·g (CV% of 63.8%) for dose-normalized total and free MPA AUC0–2, respectively. It is in accordance with other pharmacokinetic studies carried out in LN [2, 7, 20]. Recently, Abd Rahman et al. [2] reported CV% of 50% and 53% for dose-normalized total and free MPA AUC0–12, respectively. Therefore, introducing TDM of MPA in LN to adjust optimal individual MMF dose seems to be a more reasonable approach than fixed-dosing procedure.
The official TDM recommendations for MMF in LN are lacking, thus PK parameter being the best predictor of MPA exposure is still to be established. Nevertheless, contrary to the renal transplant recipients due to better kidney function observed in autoimmune diseases [21, 22], in a number of PK studies conducted in non-transplant patients a significant correlation between total MPA C0 and MPA AUC0–12 was reported (r = 0.545 [23], r = 0.561 [20], r = 0.578 [21], r = 0.643 [2], r = 0.79 [24], r = 0.90 [6], r = 0.94 [22]). The recent retrospective study that evaluated the relationship between total MPA C0 and AUC0–4 has also reported significant correlation (r = 0.55) [25]. These findings are consistent with our study since weak but still statistically significant correlation between absolute as well as dose-normalized total C0 and AUC0–2 was observed (r = 0.5508 and r = 0.5699, respectively). However, it has to be noted that the magnitude of the C0-AUC correlations reported so far in LN is not satisfactory. Only two studies carried out by Lertdumrongluk et al. [6] and Mino et al. [22] (conducted in 18 and 6 LN patients respectively) reported strong correlations with r ≥ 0.90. Nevertheless, according to our study results described in “Receiver operating characteristic curves analysis,” total dose-normalized MPA C0 may be a useful parameter to distinguish between LN patients with eGFR below and above 81 mL/min. According to the ROC curve classification model, dose-normalized total C0 above 2.76 μg/mL·g may indicate patients with eGFR below 81 mL/min with a favorable diagnostic sensitivity (true positive rate) of 83.3% and diagnostic specificity (true negative rate) of 75.0%. Therefore, measurement of total MPA C0 in LN patients may be more useful and more justifiable than in renal transplantation. However, taking into account not satisfactory magnitude of the C0-AUC correlations, the reliability of C0 measurements in MMF dose optimization needs to be confirmed in larger prospective clinical studies.
Monitoring free besides total MPA concentration gives the opportunity to calculate MPA free fraction and to observe its distribution over time. It is widely recognized that MPA is strongly bound to plasma albumin (97–99%) [8, 10] which has been also proved here. A total of 59.5% measurements amounted to less than 1% of MPA free fraction, while only 2.4% samples exceeded 3% of MPA free fraction. The distribution of MPA free fraction in human plasma was very stable until 2 h after MMF intake; however, the average maximum free MPA concentration was observed 0.5 h after MMF intake (see Supplementary Fig. 1).
To verify the usefulness of free MPA monitoring a series of correlations with PK parameters and biochemical data were made. It was found that dose-normalized free MPA C0 and free MPA AUC0–2 were significantly correlated with total MPA C0 (r = 0.7909) and total MPA AUC0–2 (r = 0.6704) respectively (see Fig. 2). These results suggest that monitoring only total MPA concentrations might seem sufficient to conduct TDM, especially taking into account that free MPA concentrations should be determined by more demanding LC-MS/MS [16], which is not as easily available in every laboratory as HPLC-UV or even immunoassays [26]. However, our results have shown that there is a significant negative relationship between serum albumin concentration and MPA free fraction, similarly to the findings reported in in vitro [10], as well as in transplantation [9] studies. In our study, elevated MPA free fraction was observed in hypoalbuminemic LN patients (serum albumin below 3.5 g/dL). Atcheson et al. [9] have concluded that in renal transplant patients with abnormal MPA free fraction TDM based on total MPA monitoring is inappropriate. Also in LN patients with hypoalbuminemia monitoring free MPA may be more beneficial than standard total MPA monitoring.
Following ROC curve analysis it was possible to determine MPA free fraction level of 1.85% that could indicate hypoalbuminemic LN patients. However, despite high diagnostic specificity (true negative rate) of 89.4%, diagnostic sensitivity (true positive rate) of 35.7% was not satisfactory to make this classification model useful in clinical practice.
Conclusion
Presented study by providing new information may support formulating TDM recommendations in LN in the future. The relationship between corresponding free and total pharmacokinetic MPA parameters as well as the effect of hypoalbuminemia on MPA plasma protein binding was examined in adult LN patients. The study results suggested that TDM of MPA in LN seems to be a more reasonable approach than the standard fixed-dose protocol in optimizing MMF therapy. Although no official TDM recommendations were published so far, measurement of predose total MPA concentrations may be a possible estimation of MPA exposure, while monitoring free rather than total MPA may be more beneficial in hypoalbuminemic patients. However, it still needs to be confirmed in larger prospective clinical studies.
References
Bertsias GK, Tektonidou M, Amoura Z, Aringer M, Bajema I, Berden JH, Boletis J, Cervera R, Dorner T, Doria A, Ferrario F, Floege J, Houssiau FA, Ioannidis JP, Isenberg DA, Kallenberg CG, Lightstone L, Marks SD, Martini A, Moroni G, Neumann I, Praga M, Schneider M, Starra A, Tesar V, Vasconcelos C, van Vollenhoven RF, Zakharova H, Haubitz M, Gordon C, Jayne D, Boumpas DT (2012) Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of adult and paediatric lupus nephritis. Ann Rheum Dis 71:1771–1782. https://doi.org/10.1136/annrheumdis-2012-201940
Abd Rahman AN, Tett SE, Abdul Gafor HA, McWhinney BC, Staatz CE (2015) Exposure-effect relationship of mycophenolic acid and prednisolone in adult patients with lupus nephritis. Br J Clin Pharmacol 80:1064–1075. https://doi.org/10.1111/bcp.12678
Shaw LM, Holt DW, Oellerich M, Meiser B, Van Gelder T (2001) Current issues in therapeutic drug monitoring of mycophenolic acid: report of a roundtable discussion. Ther Drug Monit 23:305–315
Le Meur Y, Buchler M, Thierry A, Caillard S, Villemain F, Lavaud S, Etienne I, Westeel PF, Hurault de Ligny B, Rostaing L, Thervet E, Szelag JC, Rerolle JP, Rousseau A, Touchard G, Marquet P (2007) Individualized mycophenolate mofetil dosing based on drug exposure significantly improves patient outcomes after renal transplantation. Am J Transplant 7:2496–2503. https://doi.org/10.1111/j.1600-6143.2007.01983.x
Łuszczyńska P, Pawiński T (2015) Therapeutic drug monitoring of mycophenolic acid in lupus nephritis: a review of current literature. Ther Drug Monit 37:711–717. https://doi.org/10.1097/FTD.0000000000000223
Lertdumrongluk P, Somparn P, Kittanamongkolchai W, Traitanon O, Vadcharavivad S, Avihingsanon Y (2010) Pharmacokinetics of mycophenolic acid in severe lupus nephritis. Kidney Int 78:389–395. https://doi.org/10.1038/ki.2010.170
Zahr N, Arnaud L, Marquet P, Haroche J, Costedoat-Chalumeau N, Hulot JS, Funck-Brentano C, Piette JC, Amoura Z (2010) Mycophenolic acid area under the curve correlates with disease activity in lupus patients treated with mycophenolate mofetil. Arthritis Rheum 62:2047–2054. https://doi.org/10.1002/art.27495
Dasgupta A (2016) Chapter 4 - monitoring free mycophenolic acid concentration: is there any clinical advantage? In: Dasgupta MO (ed) Personalized immunosuppression in transplantation. Elsevier, San Diego, pp 83–107
Atcheson BA, Taylor PJ, Kirkpatrick CMJ, Duffull SB, Mudge DW, Pillans PI, Johnson DW, Tett SE (2004) Free mycophenolic acid should be monitored in renal transplant recipients with hypoalbuminemia. Ther Drug Monit 26:284–286
Nowak I, Shaw LM (1995) Mycophenolic acid binding to human serum albumin: characterization and relation to pharmacodynamics. Clin Chem 41:1011–1017
Joy MS, Hilliard T, Hu Y, Hogan SL, Dooley MA, Falk RJ, Smith PC (2009) Pharmacokinetics of mycophenolic acid in patients with lupus nephritis. Pharmacotherapy 29:7–16. https://doi.org/10.1592/phco.29.1.7
Weening JJ, D’Agati V, Schwartz MM (2004) The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol 15:241–250. https://doi.org/10.1097/01.asn.0000108969.21691.5d
Kuypers DR, Le Meur Y, Cantarovich M, Tredger MJ, Tett SE, Cattaneo D, Tonshoff B, Holt DW, Chapman J, Gelder T, for The Transplantation Society (TTS) Consensus Group on T DM of MPA (2010) Consensus report on therapeutic drug monitoring of mycophenolic acid in solid organ transplantation. Clin J Am Soc Nephrol 5:341–358. https://doi.org/10.2215/CJN.07111009
Shaw LM, Korecka M, van Breeman R, Nowak I, Brayman KL (1998) Analysis, pharmacokinetics and therapeutic drug monitoring of mycophenolic acid. Clin Biochem 31:323–328
Pawinski T, Luszczynska P, Durlik M, Majchrzak J, Baczkowska T, Chrzanowska M, Sobiak J, Glyda M, Kuriata-Kordek M, Kaminska D, Krajewska M, Klinger M (2013) Development and validation of limited sampling strategies for the estimation of mycophenolic acid area under the curve in adult kidney and liver transplant recipients receiving concomitant enteric-coated mycophenolate sodium and tacrolimus. Ther Drug Monit 35:760–769. https://doi.org/10.1097/FTD.0b013e31829b88f5
Łuszczyńska P, Pawiński T, Kunicki PK, Sikorska K, Marszałek R (2017) Free mycophenolic acid determination in human plasma ultrafiltrate by a validated liquid chromatography-tandem mass spectrometry method. Biomed Chromatogr 31:e3976. https://doi.org/10.1002/bmc.3976
Borchers AT, Leibushor N, Naguwa SM, Cheema GS, Shoenfeld Y, Gershwin ME (2012) Lupus nephritis: a critical review. Autoimmun Rev 12:174–194. https://doi.org/10.1016/j.autrev.2012.08.018
Hanley JA, McNeil BJ (1982) The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143:29–36. https://doi.org/10.1148/radiology.143.1.7063747
van Gelder T, Berden JH, Berger SP (2015) To TDM or not to TDM in lupus nephritis patients treated with MMF? Nephrol Dial Transplant 30:560–564. https://doi.org/10.1093/ndt/gfu184
Alexander S, Fleming DH, Mathew BS, Varughese S, Jeyaseelan V, Tamilarasi V, Jacob CK, John GT (2014) Pharmacokinetics of concentration-controlled mycophenolate mofetil in proliferative lupus nephritis: an observational cohort study. Ther Drug Monit 36:423–432. https://doi.org/10.1097/ftd.0000000000000031
Neumann I, Haidinger M, Jager H, Grutzmacher H, Griesmacher A, Muller MM, Bayer PM, Meisl FT (2003) Pharmacokinetics of mycophenolate mofetil in patients with autoimmune diseases compared renal transplant recipients. J Am Soc Nephrol 14:721–727. https://doi.org/10.1097/01.asn.0000051598.12824.da
Mino Y, Naito T, Matsushita T, Otsuka A, Ushiyama T, Ozono S, Hishida A, Kagawa Y, Kawakami J (2008) Comparison of pharmacokinetics of mycophenolic acid and its glucuronide between patients with lupus nephritis and with kidney transplantation. Ther Drug Monit 30:656–661
Neumann I, Fuhrmann H, Fang IF, Jaeger A, Bayer P, Kovarik J (2008) Association between mycophenolic acid 12-h trough levels and clinical endpoints in patients with autoimmune disease on mycophenolate mofetil. Nephrol Dial Transplant 23:3514–3520. https://doi.org/10.1093/ndt/gfn360
Daleboudt GMN, Reinders MEJ, den Hartigh J, Huizinga TWJ, Rabelink AJ, de Fijter JW, Berger SP (2013) Concentration-controlled treatment of lupus nephritis with mycophenolate mofetil. Lupus 22:171–179
Pourafshar N, Karimi A, Wen X, Sobel E, Pourafshar S, Agrawal N, Segal E, Mohandas R, Segal MS (2018) The utility of trough mycophenolic acid levels for the management of lupus nephritis. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfy026
Kunicki PK, Pawiński T, Boczek A, Waś J, Bodnar-Broniarczyk M (2015) A comparison of the immunochemical methods, PETINIA and EMIT, with that of HPLC-UV for the routine monitoring of mycophenolic acid in heart transplant patients. Ther Drug Monit 37:311–318. https://doi.org/10.1097/ftd.0000000000000151
Acknowledgements
Special acknowledgements should go to Prof. Magdalena Krajewska (Wroclaw Medical University) for coordination of patients’ participation and to Mrs. Jadwiga Majchrzak, MSc (Transplantation Institute, Medical University of Warsaw) for collection of plasma samples from patients. The authors wish to thank also Katarzyna Sikorska, Ryszard Marszałek, Monika Rużycka, Agnieszka Dobrzańska, Aneta Jankowska, Kamil Banach, and Agnieszka Kalicka for their excellent technical assistance.
Contributions of authors
PŁ made substantial contributions to the conception and design of the work, the analysis and interpretation of data as well as drafting and revising the manuscript. TP made substantial contributions to the conception and design of the work, the acquisition of data as well as revising the manuscript. PKK made substantial contributions to the analysis and interpretation of data as well as revising the manuscript. MD, HA-B, and MH made substantial contributions to the acquisition of data as well as revising the work. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
TP and PKK have been sponsored by Roche Polska Sp. z o.o. for participation in IATDMCT Congresses: Rotterdam 2015, Kyoto 2017 and Brisbane 2018. TP has received a speaker honorarium from Chiesi Poland Sp. z o.o. MD has received a speaker honorarium from Roche Polska Sp. z o.o. and Chiesi Poland Sp. z o.o. Listed above activities were not related to the study presented. PŁ, HA-B and MH declare no conflict of interest regarding the content of this article.
Ethical approval
All procedures performed in study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (the local ethics committee approval numbers: KB/174/2013 - Medical University of Warsaw, and KB-317/2015 - Wroclaw Medical University) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(PDF 66 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Łuszczyńska, P., Pawiński, T., Kunicki, P.K. et al. Pharmacokinetics of free and total mycophenolic acid in adult lupus nephritis patients—implications for therapeutic drug monitoring. Eur J Clin Pharmacol 75, 371–379 (2019). https://doi.org/10.1007/s00228-018-2599-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-018-2599-x