Abstract
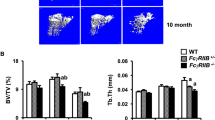
Patients with systemic lupus erythematosus (SLE), a chronic inflammatory disease characterized by loss of T- and B-cell tolerance to autoantigens, are at increased risk for osteoporosis and fractures. Mice deficient in Fc gamma receptor IIb (FcγRIIB) exhibit spontaneous SLE and its restoration rescues the disease. To determine whether deleting FcγRIIB affects cortical bone mass and mechanical properties, we analyzed cortical bone phenotype of FcγRIIB knockouts at different ages. FACS analysis revealed that 6-month-old FcγRIIB−/− mice had increased B220lowCD138+ cells, markers of plasma cells, indicating active SLE disease. In contrast, 3-month-old FcγRIIB−/− mice did not develop the active SLE disease. µCT analysis indicated that FcγRIIB deletion did not affect cortical bone in 3-month-old mutants. However, 6- and 10-month-old FcγRIIB−/− males and females had osteopenic cortical bone and the severity of bone loss increased with disease duration. FcγRIIB deletion decreased cross-sectional area, cortical area, and marrow area in 6-month-old males. Cortical area and cortical thickness were decreased in 10-month-old FcγRIIB−/− males. Lack of FcγRIIB decreased cortical thickness without affecting cortical area in females. However, deletion of a single FcγRIIB allele was insufficient to induce cortical bone loss. The bending strength was decreased in 6- and 10-month-old FcγRIIB-deficient males compared to WT controls. A microindentation analysis demonstrated significantly decreased hardness in both 10-month-old FcγRIIB−/− males and females. Our data indicate that FcγRIIB contributes to the regulation of cortical bone homeostasis subsequent to SLE development and that deletion of FcγRIIB in mice leads to SLE-like disease associated with cortical bone loss and decreased bending strength and hardness.





Similar content being viewed by others
References
Yacoub Wasef SZ (2004) Gender differences in systemic lupus erythematosus. Gend Med 1(1):12–17. https://doi.org/10.1016/S1550-8579(04)80006-8
Schwartzman-Morris J, Putterman C (2012) Gender differences in the pathogenesis and outcome of lupus and of lupus nephritis. Clin Dev Immunol 2012:604892. https://doi.org/10.1155/2012/604892
Crosslin KL, Wiginton KL (2011) Sex differences in disease severity among patients with systemic lupus erythematosus. Gend Med 8(6):365–371. https://doi.org/10.1016/j.genm.2011.10.003
de Carvalho JF, do Nascimento AP, Testagrossa LA, Barros RT, Bonfa E (2010) Male gender results in more severe lupus nephritis. Rheumatol Int 30(10):1311–1315. https://doi.org/10.1007/s00296-009-1151-9
Andrade RM, Alarcon GS, Fernandez M, Apte M, Vila LM, Reveille JD (2007) Accelerated damage accrual among men with systemic lupus erythematosus: XLIV. Results from a multiethnic US cohort. Arthritis Rheum 56(2):622–630. https://doi.org/10.1002/art.22375
Zhu TY, Griffith JF, Au SK, Tang XL, Kwok AW, Leung PC, Li EK, Tam LS (2014) Bone mineral density change in systemic lupus erythematosus: a 5-year followup study. J Rheumatol 41(10):1990–1997. https://doi.org/10.3899/jrheum.131190
Bultink IE, Lems WF, Kostense PJ, Dijkmans BA, Voskuyl AE (2005) Prevalence of and risk factors for low bone mineral density and vertebral fractures in patients with systemic lupus erythematosus. Arthritis Rheum 52(7):2044–2050. https://doi.org/10.1002/art.21110
Garcia-Carrasco M, Mendoza-Pinto C, Escarcega RO, Jimenez-Hernandez M, Etchegaray Morales I, Munguia Realpozo P, Rebollo-Vazquez J, Soto-Vega E, Deleze M, Cervera R (2009) Osteoporosis in patients with systemic lupus erythematosus. Isr Med Assoc J 11(8):486–491
Dominiak B, Oxberry W, Chen P (2005) Study on a nonhealing fracture from a patient with systemic lupus erythematosus and its pathogenetic mechanisms. Ultrastruct Pathol 29(2):107–120
Zhao R (2012) Immune regulation of osteoclast function in postmenopausal osteoporosis: a critical interdisciplinary perspective. Int J Med Sci 9(9):825–832. https://doi.org/10.7150/ijms.5180
Lee YM, Fujikado N, Manaka H, Yasuda H, Iwakura Y (2010) IL-1 plays an important role in the bone metabolism under physiological conditions. Int Immunol 22(10):805–816. https://doi.org/10.1093/intimm/dxq431
Umare V, Pradhan V, Nadkar M, Rajadhyaksha A, Patwardhan M, Ghosh KK, Nadkarni AH (2014) Effect of proinflammatory cytokines (IL-6, TNF-alpha, and IL-1beta) on clinical manifestations in Indian SLE patients. Mediat Inflamm 2014:385297. https://doi.org/10.1155/2014/385297
Li X, Liu L, Meng D, Wang D, Zhang J, Shi D, Liu H, Xu H, Lu L, Sun L (2012) Enhanced apoptosis and senescence of bone-marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Stem Cells Dev 21(13):2387–2394. https://doi.org/10.1089/scd.2011.0447
Sen D, Keen RW (2001) Osteoporosis in systemic lupus erythematosus: prevention and treatment. Lupus 10(3):227–232. https://doi.org/10.1191/096120301671413439
Bruhns P, Jonsson F (2015) Mouse and human FcR effector functions. Immunol Rev 268(1):25–51. https://doi.org/10.1111/imr.12350
Smith KG, Clatworthy MR (2010) FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol 10(5):328–343. https://doi.org/10.1038/nri2762
Bolland S, Ravetch JV (2000) Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity 13(2):277–285
McGaha TL, Sorrentino B, Ravetch JV (2005) Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science 307(5709):590–593. https://doi.org/10.1126/science.1105160
Yuasa T, Kubo S, Yoshino T, Ujike A, Matsumura K, Ono M, Ravetch JV, Takai T (1999) Deletion of fcgamma receptor IIB renders H-2(b) mice susceptible to collagen-induced arthritis. J Exp Med 189(1):187–194
Bagi CM, Hanson N, Andresen C, Pero R, Lariviere R, Turner CH, Laib A (2006) The use of micro-CT to evaluate cortical bone geometry and strength in nude rats: correlation with mechanical testing, pQCT and DXA. Bone 38(1):136–144. https://doi.org/10.1016/j.bone.2005.07.028
Su K, Yang H, Li X, Li X, Gibson AW, Cafardi JM, Zhou T, Edberg JC, Kimberly RP (2007) Expression profile of FcgammaRIIb on leukocytes and its dysregulation in systemic lupus erythematosus. J Immunol 178(5):3272–3280
Espeli M, Smith KG, Clatworthy MR (2016) FcgammaRIIB and autoimmunity. Immunol Rev 269(1):194–211. https://doi.org/10.1111/imr.12368
Seeling M, Nimmerjahn F (2015) Unlocking the bone: Fcgamma-receptors and antibody glycosylation are keys to connecting bone homeostasis to humoral immunity. Ann Transl Med 3(12):163. https://doi.org/10.3978/j.issn.2305-5839.2015.06.26
Negishi-Koga T, Gober HJ, Sumiya E, Komatsu N, Okamoto K, Sawa S, Suematsu A, Suda T, Sato K, Takai T, Takayanagi H (2015) Immune complexes regulate bone metabolism through FcRgamma signalling. Nat Commun 6:6637. https://doi.org/10.1038/ncomms7637
Su K, Wu J, Edberg JC, Li X, Ferguson P, Cooper GS, Langefeld CD, Kimberly RP (2004) A promoter haplotype of the immunoreceptor tyrosine-based inhibitory motif-bearing FcgammaRIIb alters receptor expression and associates with autoimmunity. I. Regulatory FCGR2B polymorphisms and their association with systemic lupus erythematosus. J Immunol 172(11):7186–7191
Bolland S, Yim YS, Tus K, Wakeland EK, Ravetch JV (2002) Genetic modifiers of systemic lupus erythematosus in FcgammaRIIB(-/-) mice. J Exp Med 195(9):1167–1174
Clynes R, Maizes JS, Guinamard R, Ono M, Takai T, Ravetch JV (1999) Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. J Exp Med 189(1):179–185
Kleinau S, Martinsson P, Heyman B (2000) Induction and suppression of collagen-induced arthritis is dependent on distinct fcgamma receptors. J Exp Med 191(9):1611–1616
Suzuki Y, Shirato I, Okumura K, Ravetch JV, Takai T, Tomino Y, Ra C (1998) Distinct contribution of Fc receptors and angiotensin II-dependent pathways in anti-GBM glomerulonephritis. Kidney Int 54(4):1166–1174. https://doi.org/10.1046/j.1523-1755.1998.00108.x
Ogbe A, Miao T, Symonds AL, Omodho B, Singh R, Bhullar P, Li S, Wang P (2015) Early growth response genes 2 and 3 regulate the expression of Bcl6 and differentiation of T follicular helper cells. J Biol Chem 290(33):20455–20465. https://doi.org/10.1074/jbc.M114.634816
Erickson LD, Lin LL, Duan B, Morel L, Noelle RJ (2003) A genetic lesion that arrests plasma cell homing to the bone marrow. Proc Natl Acad Sci USA 100(22):12905–12910. https://doi.org/10.1073/pnas.2131686100
Jardinet D, Lefebvre C, Depresseux G, Lambert M, Devogelaer JP, Houssiau FA (2000) Longitudinal analysis of bone mineral density in pre-menopausal female systemic lupus erythematosus patients: deleterious role of glucocorticoid therapy at the lumbar spine. Rheumatology 39(4):389–392
Lee C, Almagor O, Dunlop DD, Manzi S, Spies S, Chadha AB, Ramsey-Goldman R (2006) Disease damage and low bone mineral density: an analysis of women with systemic lupus erythematosus ever and never receiving corticosteroids. Rheumatology 45(1):53–60. https://doi.org/10.1093/rheumatology/kei079
Sels F, Dequeker J, Verwilghen J, Mbuyi-Muamba JM (1996) SLE and osteoporosis: dependence and/or independence on glucocorticoids. Lupus 5(2):89–92
Bultink IE (2012) Osteoporosis and fractures in systemic lupus erythematosus. Arthritis Care Res 64(1):2–8. https://doi.org/10.1002/acr.20568
Svenungsson E, Fei GZ, Jensen-Urstad K, de Faire U, Hamsten A, Frostegard J (2003) TNF-alpha: a link between hypertriglyceridaemia and inflammation in SLE patients with cardiovascular disease. Lupus 12(6):454–461. https://doi.org/10.1191/0961203303lu412oa
Frostegard J, Svenungsson E, Wu R, Gunnarsson I, Lundberg IE, Klareskog L, Horkko S, Witztum JL (2005) Lipid peroxidation is enhanced in patients with systemic lupus erythematosus and is associated with arterial and renal disease manifestations. Arthritis Rheum 52(1):192–200. https://doi.org/10.1002/art.20780
Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL (2000) TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Investig 106(12):1481–1488. https://doi.org/10.1172/JCI11176
Onoe Y, Miyaura C, Ito M, Ohta H, Nozawa S, Suda T (2000) Comparative effects of estrogen and raloxifene on B lymphopoiesis and bone loss induced by sex steroid deficiency in mice. J Bone Miner Res 15(3):541–549. https://doi.org/10.1359/jbmr.2000.15.3.541
Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian WP, Weitzmann MN (2007) B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood 109(9):3839–3848. https://doi.org/10.1182/blood-2006-07-037994
Alele JD, Kamen DL, Hunt KJ, Ramsey-Goldman R (2011) Bone geometry profiles in women with and without SLE. J Bone Min Res 26(11):2719–2726. https://doi.org/10.1002/jbmr.466
Acknowledgements
We thank Anucharte Srijunbarl for his suggestion about microindentation and Dr. Kevin Tompkins for his critical reading of the manuscript.
Funding
This research was funded by the Ratchadapisek Sompoch Endowment Fund (2014), Chulalongkorn University (CU-57-091-IC), and the Faculty of Dentistry, Chulalongkorn University (DRF 61019) to S. Lotinun.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Worasit Saiworn, Arthid Thim-uam, Peerapat Visitchanakun, Korakot Atjanasuppat, Jiratha Chantaraaumporn, Jutarat Mokdara, Sirintra Chungchatupornchai, Prapaporn Pisitkun, Asada Leelahavanichkul, Suchit Poolthong, Roland Baron, and Sutada Lotinun declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
The animals were housed at the Faculty of Medicine, Chulalongkorn University and maintained in accordance with the Guide for the Care and Use of Laboratory Animals (eight edition), National Research Council. All procedures were approved by the Institutional Animal Care and Use Committee at Faculty of Medicine, Chulalongkorn University.
Rights and permissions
About this article
Cite this article
Saiworn, W., Thim-uam, A., Visitchanakun, P. et al. Cortical Bone Loss in a Spontaneous Murine Model of Systemic Lupus Erythematosus. Calcif Tissue Int 103, 686–697 (2018). https://doi.org/10.1007/s00223-018-0464-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-018-0464-7