Abstract
Vitamin D insufficiency is common in patients with osteoporosis. We conducted a randomized trial comparing alendronate 70 mg combined with vitamin D3 5,600 IU in a single tablet (ALN/D5600, n = 257) with standard care chosen by the patients’ personal physicians (n = 258) in patients with postmenopausal osteoporosis (BMD T score ≤2.5 or ≤1.5 and a prior fragility fracture) who had vitamin D insufficiency (serum 25[OH]D values 8–20 ng/ml) and who were at risk of falls. Virtually all patients randomized to standard care received bisphosphonate therapy, and in approximately 70% of cases this was combined with vitamin D supplements. However, only 24% took ≥800 IU/day of supplemental vitamin D. At 6 months the proportion of patients with vitamin D insufficiency was 8.6% in the ALN/D5600 group compared with 31.0% in the standard care group (P < 0.001). Those in the ALN/D5600 group also had a greater reduction in urinary NTX/creatinine ratio (−57% vs. −46%, P < 0.001) and bone-specific alkaline phosphatase (−47% vs. −40%, P < 0.001). In the ALN/5600 group, by 12 months the increase in BMD was greater at the lumbar spine (4.9% vs. 3.9%, P = 0.047) and the total hip (2.2% vs. 1.4%, P = 0.035), significantly fewer patients were vitamin D—insufficient (11.3% vs. 36.9%, P < 0.001), and bone turnover marker (BTM) results were similar to those at 6 months. There was no difference between groups in those who experienced falls or fractures, and adverse events were similar. Based on the finding that ALN/D5600 was more effective than standard care at correcting vitamin D insufficiency, increasing BMD, and reducing BTMs in this patient group, greater attention needs to be directed toward optimizing the treatment of osteoporosis and correcting vitamin D deficiency in postmenopausal women.
Similar content being viewed by others
Vitamin D deficiency and insufficiency are prevalent worldwide, especially among osteoporotic women [1]. Although there is continued debate as to what constitutes an optimal serum concentration of 25-hydroxyvitamin D (25[OH]D), vitamin D deficiency is often defined as a value <8 ng/ml (20 nmol/l). There is a consensus that serum 25(OH)D concentrations should be >20 ng/ml (50 nmol/l) for optimal bone health [2], whereas some have suggested that values of 30 ng/ml (75 nmol/l) may be preferable [3]. At the present time, however, patients whose serum 25(OH)D concentrations are above the limit for deficiency (8 ng/ml) but below the level for optimal bone health (20 ng/ml) are termed to have “vitamin D insufficiency.”
One multinational study has shown that the average concentration of 25(OH)D in postmenopausal women is 12 ng/ml [4]. Another study reported that about 50% of women receiving therapy for osteoporosis had 25(OH)D concentrations lower than 30 ng/ml but that 25% had levels lower than 20 ng/ml [5]. The risk factors for vitamin D deficiency include low dietary vitamin D, older age, obesity, and low sun exposure [1, 5], but epidemiologic studies have revealed that there is only a weak correlation between serum 25(OH)D and latitude in osteoporotic subjects [6]. Furthermore, vitamin D deficiency and insufficiency can occur among individuals who have apparently had abundant sunlight exposure [7]. Previous studies have shown that serum 25(OH)D levels correlate inversely with PTH and bone turnover markers (BTMs) but positively with bone mineral density (BMD) [6, 8–12]. In one study, concentrations of 25(OH)D at or below 12 ng/ml were also associated with an increased risk for osteoporotic fracture in older individuals [13]. There is ample evidence to demonstrate that vitamin D supplementation, with or without calcium, leads to a decrease in PTH [8, 14, 15], suppression of BTMs [15, 16], and an increase in BMD [10, 14]. In addition, vitamin D supplementation prevents falls in older adults [17]. There is also evidence to suggest that vitamin D reduces fracture risk, with antifracture efficacy best documented in older individuals receiving vitamin D3 supplementation at 700–800 international units (IU) per day [18].
Calcium and vitamin D supplements are widely prescribed as an adjunct to other osteoporosis treatments. Unfortunately, adherence to these supplements is poor [19–21], despite the fact that coadministration of vitamin D with antiosteoporosis therapy enhances the therapeutic response [10, 22]. In addition, the doses prescribed are often lower than current recommendations. A combination tablet of alendronate 70 mg plus vitamin D3 5,600 IU (ALN/D5600) for weekly administration was formulated in order to address this issue and improve vitamin D status in patients receiving alendronate therapy. In this study we evaluated the efficacy and safety of ALN/D5600 in women with osteoporosis who had vitamin D insufficiency (serum 25[OH]D values 8–20 ng/ml) and who were at increased risk of falls. This was done within the context of a randomized controlled trial in which one group of patients received ALN/D5600 and the other received “standard care” for osteoporosis using a similar design to that used previously in the treatment of hypertension [23]. In the context of this study, patients in the standard-care group were referred to primary-care physicians or other specialists for treatment that could include any appropriate and locally available option. These physicians decided on treatment independently of the investigators without knowledge about the trial design and study end points.
Methods
Study Design
This was a multicenter, international, randomized trial using referred care as active control. Participants were randomized—using a computer-generated allocation schedule generated by the study sponsor—to treatment with the weekly ALN/D5600 combination tablet under the care of a study investigator or referral to a primary-care physician or specialist, who was not an investigator, for usual care of osteoporosis and low vitamin D. In countries where osteoporosis treatment was not reimbursed, the study sponsor provided financial aid. All participants visited investigator sites periodically for assessments of efficacy and safety. The study was conducted over a 12-month period. Due to the referred-care nature of the trial, it was an open-label design. However, participants, investigators, site staff, and the sponsor’s clinical team were blinded to postbaseline concentrations of serum 25(OH)D and BTMs. An exception to this was serum 25(OH)D <6 ng/ml, identified by the central laboratory. When this occurred, “rescue” vitamin D was administered and study treatment was discontinued.
Participants
Women who participated in this trial were ≥65 years of age with BMD T scores ≤−2.5 at the lumbar spine, total hip, or femoral neck or a T score of ≤−1.5 plus a previous fragility fracture of the hip, spine, wrist, humerus, or clavicle. All participants were postmenopausal, defined as ≥1 year without menses, ≥6 months without menses with serum FSH levels >40 mIU/ml, or ≥6 months without menses following bilateral oophorectomy. Participants also had vitamin D insufficiency (serum 25[OH]D concentrations 8–20 ng/ml) and were at increased risk of falls defined by the fact that they had suffered one or more falls within 12 months before study initiation and had reduced lower extremity physical function (score 4–9 on the Short Physical Performance Battery [SPPB]) [24, 25]. Among exclusion criteria were use of active hormonal vitamin D analogs within 2 months, oral bisphosphonates for ≥2 months within the previous 2 years, IV pamidronate or ibandronate within the previous year, fluoride treatment at >1 mg/day for over 2 weeks within the past 3 months, strontium-containing products for more than 2 weeks within the past 6 months, parathyroid hormone for more than 2 weeks within the past 3 months, anabolic steroids within the past 12 months, systemic glucocorticoids (≥5 mg/day prednisone or equivalent) for more than 2 weeks within the past 6 months, any use of IV zoledronate, current use of chemotherapy or heparin, need for assistance walking or standing up, abnormal laboratory safety screening tests or electrocardiogram, malignancy within the previous 5 years, malabsorption syndrome, or uncontrolled upper gastrointestinal disorders, cardiovascular disorders, primary or secondary hyperparathyroidism, thyroid disease, or renal disease. All participants provided written informed consent. The study was conducted in accordance with the principles of good clinical practice and approved by the appropriate institutional review boards and regulatory agencies.
Assessments
Biochemical Assessments
Blood and urine samples were collected at screening, baseline, and months 3, 6, and 12 and analyzed at Quest Diagnostics (Valencia, CA). Serum 25(OH)D samples were analyzed by a high-performance liquid chromatography/tandem mass spectrometry assay. It should be noted that the assay was recalibrated after recruitment of participants. However, baseline and on-treatment 25(OH)D measurements were all performed with the second, recalibrated assay, with calibration verified by comparison to National Institute of Standards Technology 25(OH)D standards. BTMs were also analyzed by Quest Diagnostics. Urinary N-telopeptides of type I collagen (NTX) concentration was determined using a competitive immunoassay technique, and serum bone-specific alkaline phosphate (BSAP) concentration was determined using an immunoenzymatic assay technique.
BMD
BMD was measured at screening and month 12 using dual-energy X-ray absorptiometry (DXA). The same DXA scanner was used for each participant’s measurements.
Falls
Participants were asked to record falls on a study calendar and to notify the study site of falls by telephone or prestamped postcard. After notification, study-site personnel telephoned women reporting falls to obtain details about the event, including a description of the fall, the location, contributing factors (e.g., tripping, poor vision), the immediate aftermath, longer-term consequences, and the medical attention that was required. All participants were also routinely contacted on a monthly basis to survey fall incidences (except when an office visit was scheduled in the same month, where fall events were reviewed). Falls were adjudicated by an independent committee, blinded to treatment allocation. During the study a fall was defined as “coming to rest unintentionally on the ground or other lower level from a standing or sitting position, not due to major intrinsic or extrinsic event.”
Adverse Experiences
Reports of clinical and laboratory adverse events (AEs) were collected at physician visits at months 3, 6, and 12. Participants could report an AE at any time throughout the trial.
Statistical Methods
The primary end point of the trial was the proportion of patients with serum 25(OH)D <20 ng/ml after 6 months of treatment. Secondary end points were the proportion of patients with 25(OH)D <20 ng/ml at 12 months, percent changes in the BTMs NTX (expressed as a ratio to urinary creatinine) and BSAP at 6 and 12 months, and percent changes in lumbar spine and total-hip BMD at 12 months. Exploratory end points included the incidence of falls during 12 months of treatment and change from baseline in 25(OH)D. Data were analyzed by a modified intent-to-treat approach: Women who took at least one dose of ALN/D5600 or were assigned to receive usual care and had data reported at least once postrandomization were included in the efficacy and safety analyses. For analysis of the fall-related end points, all randomized patients were included. A per-protocol analysis was performed for BTMs, to describe changes observed during the actual use of specific drugs and nutrients. Between-group differences in the primary end point were assessed using a logistic regression model, entering treatment, baseline 25(OH)D stratum (≤15 ng/ml or >15 ng/ml), baseline age stratum (≤75 or >75 years), and region (North America and Europe or the rest of the world) into the model. A log transformation was applied to NTX and BSAP before the calculation of percent change from baseline using a longitudinal data-analysis method [26], including terms for treatment, time, interaction of time by treatment, as well as adjustment for baseline 25(OH)D stratum, region, and age stratum. A similar method was used for calculating change from baseline in 25(OH)D. For percent change from baseline in lumbar spine and total-hip BMD at 12 months, a traditional longitudinal data-analysis model was applied with terms for treatment, baseline 25(OH)D stratum, region, and age stratum. Intake of vitamin D supplements was calculated, using concomitant medicine reports, by summing the intake of all vitamin D analogs for each participant and dividing the amount consumed by the days on therapy to calculate units per day of the 1-year study. The effect on falls was evaluated using a Cox proportional hazards model for time to first or second fall. Missing data were not imputed. Safety and tolerability were assessed in all patients as treated, and the analysis is descriptive.
Results
Participants and Treatment
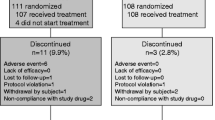
The disposition of the participants in the trial is shown in Fig. 1. Women were recruited from 77 centers in 24 countries throughout the world between July 2008 and July 2010, and most participants (88.5%) completed the 1 year of treatment. The treatment groups were well matched at baseline (Table 1). The average age of participants was 73 years, and 72% were Caucasian. All women had 25(OH)D <20 ng/ml at screening, and the average 25(OH)D was 14.9 ng/ml; but at baseline approximately 84% of women had 25(OH)D concentrations <20 ng/ml. Among the referred-care patients, almost all were prescribed treatments for osteoporosis (Table 2A). Treatments included bisphosphonates (alendronate, risedronate, ibandronate, or zoledronate), strontium, hormonal vitamin D, raloxifene, or supplements alone. Approximately 71% of the women who received active drugs also received instructions to take calcium and/or vitamin D supplementation. The estimated vitamin D intake in the referred-care group is summarized in Table 2B. Only 24.1% of the women in referred care took ≥800 IU vitamin D/day.
Serum 25(OH)D
Over three times as many women in the referred-care treatment group compared with the ALN/D5600 treatment group had 25(OH)D concentrations <20 ng/ml. At 6 months 8.6% of participants in the ALN/D5600 group and 31.0% in the referred-care group had concentrations of 25(OH)D <20 ng/ml (P < 0.001) (Fig. 2a). Similar findings were observed at 12 months (11.3 and 36.9% for ALN/D5600 and referred care, respectively, P < 0001). The proportions of participants with 25(OH)D concentrations below various threshold levels at 12 months is shown in Fig. 2b. At 12 months, the mean (95% confidence interval [CI]) serum 25(OH)D concentrations in the ALN/D5600 group had risen by 12.7 (11.4–14.0) ng/ml compared with 8.4 (7.1–9.7) ng/ml in the referred-care group. The treatment difference was 4.3 (2.5–6.1) ng/ml (Fig. 3a). Figure 3b, c illustrate the distribution of 25(OH)D values in each treatment group.
Concentrations of serum 25(OH)D. a Changes from baseline in 25(OH)D. b, c Distribution of 25(OH)D concentrations after treatment with ALN/D5600 or referred care at 6 and 12 months. Boxes represent the borders of the 25th and 75th percentiles. Center lines in boxes designate the median and points in boxes, the mean. Lines at the tops and bottoms (“whiskers”) represent the 2.5th and 97.5th percentiles
BTMs
BTMs were measured at 6 and 12 months. Concentrations of both markers decreased in each treatment group (Fig. 4a, b), and the reduction was significantly greater in the ALN/D5600 group (P < 0.001 for both markers).
BMD
At the end of 1 year, BMD was also measured. Again, both treatment groups showed significant improvement in BMD, and the increases at the spine and hip were significantly greater in the ALN/D5600 group than the referred-care group (P = 0.047 at the lumbar spine, P = 0.035 at the total hip, Fig. 4c).
Falls
There was no significant difference between treatment groups in the number of women who had at least one or at least two falls during the 12 months of the trial (Fig. 5). The hazard ratios (95% CI) of ALN/D5600 compared with referred care were 0.82 (0.59–1.15) for time to first fall and 0.93 (0.55–1.59) for time to second fall. Analysis using zero-inflated Poisson regression of fall rates gave similar results.
Safety and Tolerability
There were no differences in tolerability between the two treatment groups (Table 3). The incidences of specific AEs, including fractures, dental disorders, and upper respiratory disturbances, were similar between groups.
Discussion
This study has shown that administration of ALN/D5600 is more effective than standard care at elevating serum 25(OH)D values, reducing BTMs, and increasing lumbar spine and total hip BMD. Significantly fewer women in the ALN/D5600 group had 25(OH)D levels <20 ng/ml compared with the standard-care group (8.6% vs. 31.0%). Similarly, the reduction in BTMs was significantly greater in the ALN/D5600 group and the responses of lumbar spine and total-hip BMD were superior, even though most of the women in the referred-care group were prescribed bisphosphonates (predominantly alendronate), along with calcium and vitamin D.
The greater efficacy in the ALN/D5600 group is likely to be the result of several factors. While 51% of the referred-care group were prescribed alendronate, a substantial proportion were prescribed risedronate, which is a less potent antiresorptive than alendronate [27]. Smaller numbers of patients received strontium ranelate and raloxifene, which are relatively weak antiresorptives; and a few patients received calcium and vitamin D supplements alone. Although most patients in the standard-care group were prescribed calcium and vitamin D supplements, the estimated vitamin D intake was lower than that in the ALN/D5600 group. Although we did not collect data on adherence to calcium and vitamin D supplements in the standard-care group, previous studies have shown that many patients prescribed calcium and vitamin D supplements do not adhere to this medication [19–21]. Despite the fact that the biochemical markers and BMD responses were better in the ALN/D5600 group, we did not observe a difference in the incidence of falls or fractures between the treatment groups, although the study had insufficient power to detect effects on falls or fractures.
In summary, the present study has demonstrated that treatment of postmenopausal osteoporotic women with a combination of alendronate and vitamin D3 5600 IU is more effective than standard care at correcting vitamin D insufficiency, most probably because the vitamin D is given at the same time as the antiresorptive therapy, without the need for an additional prescription. It is important to note that in these patients with vitamin D insufficiency, even in the ALN/D5600 group, approximately two-thirds of patients did not attain serum 25(OH)D levels >30 ng/ml. This indicates that vitamin D3 doses in excess of 800 IU daily may be required to ensure that higher levels of 25(OH)D advocated by some are achieved in this population. In view of the fact that adherence remains an important issue with calcium and vitamin D supplements, it is possible that combinations of specific antiosteoporotic agents with vitamin D, such as that described here, might give improved 25(OH)D status as well as BTMs and BMD in osteoporotic patients at risk of vitamin D deficiency. Furthermore, this study suggests there is room for improvement in the usual care of postmenopausal women with osteoporosis.
References
Gaugris S, Heaney RP, Boonen S, Kurth H, Bentkover JD, Sen SS (2005) Vitamin D inadequacy among post-menopausal women: a systematic review. QJM 98:667–676
Norman AW, Bouillon R, Whiting SJ, Vieth R, Lips P (2007) 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol 103:204–205
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357:266–281
Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, Ragi-Eis S, Chandler J (2006) The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med 260:245–254
Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE (2005) Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 90:3215–3224
Kuchuk NO, van Schoor NM, Pluijm SM, Chines A, Lips P (2009) Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: global perspective. J Bone Miner Res 24:693–701
Binkley N, Novotny R, Krueger D, Kawahara T, Daida YG, Lensmeyer G, Hollis BW, Drezner MK (2007) Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab 92:2130–2135
Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T (2001) A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the Multiple Outcomes of Raloxifene Evaluation clinical trial. J Clin Endocrinol Metab 86:1212–1221
Bischoff-Ferrari HA, Dietrich T, Orav EJ, Dawson-Hughes B (2004) Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med 116:634–639
Geller JL, Hu B, Reed S, Mirocha J, Adams JS (2008) Increase in bone mass after correction of vitamin D insufficiency in bisphosphonate-treated patients. Endocr Pract 14:293–297
Kamel S, Brazier M, Rogez JC, Vincent O, Maamer M, Desmet G, Sebert JL (1996) Different responses of free and peptide-bound cross-links to vitamin D and calcium supplementation in elderly women with vitamin D insufficiency. J Clin Endocrinol Metab 81:3717–3721
Lips P (2001) Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 22:477–501
van Schoor NM, Visser M, Pluijm SM, Kuchuk N, Smit JH, Lips P (2008) Vitamin D deficiency as a risk factor for osteoporotic fractures. Bone 42:260–266
Ooms ME, Roos JC, Bezemer PD, van der Vijgh WJ, Bouter LM, Lips P (1995) Prevention of bone loss by vitamin D supplementation in elderly women: a randomized double-blind trial. J Clin Endocrinol Metab 80:1052–1058
Prestwood KM, Pannullo AM, Kenny AM, Pilbeam CC, Raisz LG (1996) The effect of a short course of calcium and vitamin D on bone turnover in older women. Osteoporos Int 6:314–319
von Hurst PR, Stonehouse W, Kruger MC, Coad J (2010) Vitamin D supplementation suppresses age-induced bone turnover in older women who are vitamin D deficient. J Steroid Biochem Mol Biol 121:293–296
Bischoff-Ferrari H, Dawson-Hughes B, Staehelin H, Orav J, Stuck A, Theiler R, Wong J, Egli A, Kiel D, Henschkowski J (2009) Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ 339:b3692
Boonen S, Lips P, Bouillon R, Bischoff-Ferrari HA, Vanderschueren D, Haentjens P (2007) Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: evidence from a comparative meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 92:1415–1423
Segal E, Zinman C, Raz B, Ish-Shalom S (2009) Low patient compliance—a major negative factor in achieving vitamin D adequacy in elderly hip fracture patients supplemented with 800 IU of vitamin D3 daily. Arch Gerontol Geriatr 49:364–367
Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, Bassford T, Beresford SA, Black HR, Blanchette P, Bonds DE, Brunner RL, Brzyski RG, Caan B, Cauley JA, Chlebowski RT, Cummings SR, Granek I, Hays J, Heiss G, Hendrix SL, Howard BV, Hsia J, Hubbell FA, Johnson KC, Judd H, Kotchen JM, Kuller LH, Langer RD, Lasser NL, Limacher MC, Ludlam S, Manson JE, Margolis KL, McGowan J, Ockene JK, O’Sullivan MJ, Phillips L, Prentice RL, Sarto GE, Stefanick ML, Van Horn L, Wactawski-Wende J, Whitlock E, Anderson GL, Assaf AR, Barad D (2006) Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med 354:669–683
Grant AM, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, Anderson FH, Cooper C, Francis RM, Donaldson C, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA (2005) Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet 365:1621–1628
Adami S, Giannini S, Bianchi G, Sinigaglia L, Di Munno O, Fiore CE, Minisola S, Rossini M (2009) Vitamin D status and response to treatment in post-menopausal osteoporosis. Osteoporos Int 20:239–244
Writing Committee on Behalf of the HDFP Cooperative Group (1976) The Hypertension Detection and Follow-Up Program. Prev Med 5:207–215
Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB (1995) Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 332:556–561
Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB (2000) Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 55:M221–M231
Liang K-Y, Zeger SL (2000) Longitudinal data analysis of continuous and discrete response for pre–post designs. Indian J Stat 62:134–148
Bonnick S, Saag KG, Kiel DP, McClung M, Hochberg M, Burnett SM, Sebba A, Kagan R, Chen E, Thompson DE, de Papp AE (2006) Comparison of weekly treatment of postmenopausal osteoporosis with alendronate versus risedronate over two years. J Clin Endocrinol Metab 91:2631–2637
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
This study was sponsored by Merck. S. Ralston has received consultancy fees from Merck Sharp & Dohme, a subsidiary of Merck & Co., Inc. N. Binkley has received consultancy fees from Merck Sharp & Dohme, a subsidiary of Merck & Co., Inc. S. Boonen has received research grants and consultancy fees from Merck Sharp & Dohme, a subsidiary of Merck & Co., Inc. D. Kiel has received research grants and consultancy fees from Merck Sharp & Dohme, a subsidiary of Merck & Co., Inc. J. Reginster has received consultancy fees from Merck Sharp & Dohme, a subsidiary of Merck & Co., Inc. C. Roux received honoraria and/or lecture fees and/or research grants from Merck Sharp & Dohme, a subsidiary of Merck & Co., Inc. L. Chen, E. Rosenberg, and A. Santora are employees of Merck & Co., Inc. and may own stock in the company.
Appendix: FOCUS-D Investigators
Appendix: FOCUS-D Investigators
Tara Coughlan, Adelaide & Meath Hospital, Dublin, Ireland; Asma Arabi, American University of Beirut Medical Center, Beirut, Lebanon; Ayse Kucukdeveci, Ankara University, Ankara, Turkey; Naren Savani, Batchworth House Mount Vernon Hospital, Middlesex, UK; Rasa Kausiene, Bendrosios terapijos skyrius, Siauliai, Lithuania; Bruno Pornel, Brussels Menopause Center, Brussels, Belgium; Tom Price, Cannock Chase Hospital, Staffordshire, UK; Claude-Laurent Benhamou, Centre Hop Porte Madeleine, Orleans, France; Maria Aguilar, Centro de Diagnostico Medicont S.A. de C.V., Nezahualcoyotl, Mexico; Mauricio Abello, Centro Integral de Reumatologia del Caribe, Barranquilla, Atlantico, Colombia; Elgardo Tobias, Centro Integral de Reumatologia e Inmunologia, Bogota, Cundinamarca, Colombia; Nadeem Rais, Chowpatty Medical Center, Mumbai, Maharashtra, India; Olga Ershova, Clinical Hospital for Emergency Care n.a. Soloviev, Yaroslavl, Russian Federation; Rene Martz, Clinical Research Hamburg, Hamburg, Germany; Sultan Linjawi, Coffs Endocrine & Diabetes Services, Coffs Harbour, NSW, Australia; Stuart Kramer, Danville Orthopedic Clinic, Danville, VA, USA; Jesus Walliser, Eentro Walliser de Osteoporosis y Metabolismo Mineral, Veracruz, Mexico; Simin Hepguler, Ege University, Bornova, Turkey; Jeffrey Gimble, Family Health Center, Baton Rouge, LA, USA; Olga Lesnyak, Family Medicine Clinic of Ural State Medical Academy, Ekaterinburg, Russian Federation; Philippe Chalem, Fundacion Instituto de Reumatologia Fernando Chalem, Bogota, Colombia; Jose Aramburu, H. de Basurto, Bilbao, Vizaya, Spain; Joseph Foldes, Hadassah Har-Hatzofim Medical Center, Jerusalem, Israel; Yasser Yaghi, Hammoud Hospital, Saida South, Lebanon; Christian Roux, Hopital Cochin, Paris, France; Carlos Abud, Hospital Central Dr. Ignacio Morones Prieto, San Luis Potosi, Mexico; Manuel Muñoz Torres, Hospital Clinico San Cecilio, Granada, Spain; Gilberto González, Hospital Clinico Universidad Catolica de Chile, Santiago, Chile; Georges Weryha, Hospital de Brabois, Vandoeuvre les Nancy, France; Emilio Martín Mola, Hospital Universitario La Paz, Madrid, Spain; Marina Shargordsky, Hypertension Clinic Wolfson Medical Center, Holon, Israel; Neeta Patel Inkosi Albert Luthuli Hospital, Durban, South Africa; Kurt Lippuner, Inselspital–Poliklinik für Osteoporose, Bern, Switzerland; Ruediger Moericke, Institut für Praventive Medizin & Klinische Forschung, Magdeburg, Germany; Basel Masri, Jordan Hospital, Amman, Jordan; Inguta Stura, Juglas Medicinas Centrs, Riga, Latvia; Robert Theiler, Klinik für Rheumatologie und Rehabilitation Stadtsp., Zurich, Switzerland; Nigar Dursun, Kocaeli University, Kocaeli, Turkey; Vincent Thompson, Libra Clinical Research Associates, Brick, NJ, USA; Dac Andersone, Liepajas Metalurga Poliklinika, Liepaja, Latvia; Mark Arya, Maroubra Medical Centre, Maroubra, NSW, Australia; Carl-Peter Anderberg, Me3Plus Clinical Trials AB, Goteborg, Sweden; Michael Pfeifer, MEDWISS Bad Pyrmont, Bad Pyrmont, Germany; Hugo Goldstraj, Multispecialty Medical Center, North Miami Beach, FL, USA; Vidmantas Alekna, Nacionalinis Osteoporozes Centras, Vilnius, Lithuania; Bram Wieskopf, North Georgia Clinical Research, Woodstock, GA, USA; Tobias De Villiers, Panorama Medi Clinic, Parow, Western Cape, South Africa; Salvatore Minisola, Policlinico Umberto I Sapienza University of Rome, Rome, Italy; Jean-Yves Reginster, Polyclinique L. Brull-Exploration de l’Os et du Cartilage, Liège, Belgium; Klaus Heil, Praxis Dr. Klaus Heil, Tuttlingen, Germany; Volkmar Herkt, Praxis Dr. Volkmar Herkt, Dresden, Germany; Jane Rohlf, Premier Research, Trenton, NJ, USA; Albert De Weerd, Pretoria East Private Hospital, Gauteng, South Africa; Sophia Ish-Shalom, Rambam Medical Center, Haifa, Israel; Loreta Bukauskiene, Reumatologijos skyrius, Klaipeda, Lithuania; Lidia Benevolenskaya, Research Institute of Rheumatology, Moscow, Russian Federation; Ruben Mantilla, Riesgo de Feractura S.A., Bogota, Cundinamarca, Colombia; Anthony Woolf, Royal Cornwall Hospitals NHS Trust, Cornwall, UK; William Otero, SERVIMED Bucaramanga, Santander, Colombia; Savithree Nayiager, St. Augustine’s Hospital, Kwazulu Natal, South Africa; Bernard Walsh, St. James’ Hospital–Robert Mayne Day Hospital, Dublin, Ireland; Stephen Newman, Brick, NJ, USA; Nitin Bagul, Synexus Lancashire Clinical Research Centre, Lancashire, UK; Essam Abdulhakim, Synexus Ltd. Merseyside Clinical Research Centre, Liverpool, UK; Shilpa Govindraj, Synexus Manchester Clinical Research Centre, Manchester, UK; Rex Sarmiento Synexus Midlands Clinical Research Centre, Birmingham, UK; Rahul Ellahbadi, Synexus Scotland Clinical Research Centre, Glasgow, UK; Hilary Shaw, Synexus Thames Valley Clinical Research, Berkshire, UK; Hawys Thomas, Synexus Wales Clinical Research Centre, Cardiff, UK; Stanley Lipschitz, The Memory Centre, Johannesburg, South Africa; Sergio Gutierrez, Torre de Especialidades–Ant Hosp. Civil de Gdl, Guadalajara, Mexico; Neil Binkley, University of Wisconsin, Madison, WI, USA; Stuart Ralston, Western General Hospital, Edinburgh, UK; Michael Davey, Westville Hospital, Durban, South Africa; Renato Lauro, Azienda Ospedaliera Universitaria, Rome, Italy; Hector Rodriguez, Central Medical & Therapy Center, Miami, FL, USA; Muataz Al-Ramahi, Jordan University Hospital, Amman, Jordan.
Rights and permissions
About this article
Cite this article
Ralston, S.H., Binkley, N., Boonen, S. et al. Randomized Trial of Alendronate Plus Vitamin D3 Versus Standard Care in Osteoporotic Postmenopausal Women with Vitamin D Insufficiency. Calcif Tissue Int 88, 485–494 (2011). https://doi.org/10.1007/s00223-011-9482-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-011-9482-4