Abstract
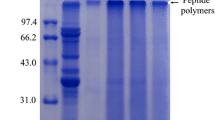
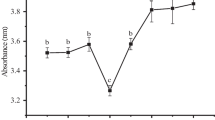
The effect of glycosylation following limited enzymatic hydrolysis on conformational and functional properties of black bean protein isolate (BBPI) was investigated in this study. The black bean protein hydrolysate (HBBPI) was prepared using alcalase at pH 9.0 and then glycosylated with glucose (G) at 80 °C for different incubation times from 1 to 6 h. Grafted HBBPI with glucose (HBBPI-G conjugates) had a higher molecular weight than HBBPI by SDS-PAGE analysis. HBBPI-G conjugates had a higher content of β-turn and random coil, but a lower content of α-helix and β-sheet structure than BBPI. HBBPI-G conjugates had lower fluorescence intensity and exhibited bathochromic shift compared with BBPI. Subsequently, the functional properties were also evaluated. Results indicated that the emulsifying activity and solubility were obviously improved (P < 0.05) by HBBPI-G conjugates incubated for 4 h with 6.5% degree of hydrolysis compared to BBPI. Additionally, the glycosylation had positive effects on the reducing power and hydroxyl radical scavenging rate. Therefore, the combination of limited hydrolysis and glycation can be used as an effective method for BBPI modification to obtain enhanced functional properties.







Similar content being viewed by others
References
Inagaki S, Morimura S, Shigematsu T, KidaI K, Akutagama H (2005) Apoptosis induction by vinegar produced from boiled extract of black soybeans in human monoblastic leukemia U937 cells: difference in sensitivity to cell toxicity compared to normal lymphocytes. Food Sci Technol Res 11(3):311–317
Takahashi R, Ohmori R, Kiyose C, Momiyama Y, Ohsuzu F, Kondo K (2005) Antioxidant activities of black and yellow soybeans against low density lipoprotein oxidation. J Age Food Chem 53(11):4578–4582
Xu B, Chang SKC (2008) Antioxidant capacity of seed coat, dehulled bean, and whole black soybeans in relation to their distributions of total phenolics, phenolic acids, anthocyanins, and isoflavones. J Age Food Chem 56(18):8365–8373
Lee HI, Hung YH, Chou CC (2008) Solid-state fermentation with fungi to enhance the antioxidative activity, total phenolic and anthocyanin contents of black bean. Int J Food Microbiol 121(2):150–156
Evangelho JA, Vanier NL, Pinto VZ, Dias AR, Zavareze Eda R (2017) Black bean (Phaseolus vulgaris L.) protein hydrolysates: physicochemical and functional properties. Food Chem 214:460–467
Zhu D, Damodaran S, Lucey JA (2008) Formation of whey protein isolate (WPI)-dextran conjugates in aqueous solutions. J Age Food Chem 56:7113–7118
Jiang LZ, Wang J, Li Y, Wang ZJ, Liang J (2014) Effects of ultrasound on the structure and physical properties of black bean protein isolates. Food Res Int 62:595–601
David B, Teresita S, Jorge R, Maira S, Luis C (2014) Enzymatic hydrolysis of hard-to-cook bean (Phaseolus vulgaris L.) protein concentrates and its effects on biological and functional properties. Int J Food Sci Technol 49(1):2–8
Chen L, Chen J, Ren J, Zhao M (2011) Modifications of soy protein isolates using combined extrusion pre-treatment and controlled enzymatic hydrolysis for improved emulsifying properties. Food Hydrocoll 25(5):887–897
Kong BH, Xiong YLL (2006) Antioxidant activity of zein hydrolysates in a liposome system and the possible mode of action. J Agric Food Chem 54(16):6059–6068
Pirestani S, Nasirpour A, Keramat J, Desobry S (2017) Preparation of chemically modified canola protein isolate with gum Arabic by means of Maillard reaction under wet-heating conditions. Carbohydr Polym 155:201–207
Zhang YT, Tan C, Zhang XM, Xia SQ, Jia CS (2014) Effects of maltodextrin glycosylation following limited enzymatic hydrolysis on the functional and conformational properties of soybean protein isolate. Eur Food Res Technol 238(6):957–968
Li Y, Zhong F, Ji W, Yokoyama W, Shoemaker CF, Zhu S, Xia W (2013) Functional properties of Maillard reaction products of rice protein hydrolysates with mono-, oligo- and polysaccharides. Food Hydrocoll 30(1):53–60
Adler-nissen J (1986) Enzymic hydrolysis of food proteins. Elsevier Applied Science Publishers, London
Evangelho JA, Vanier NL, Pinto VZ, Berrios JJ, Dias AR (2017) Black bean (Phaseolus vulgaris L.) protein hydrolysates: physicochemical and functional properties. Food Chem 214:460–467
Church FC, Swaisgood HE, Porter DH, Catignani GL (1983) Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J Dairy Sci 66(6):1219–1227
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Wang YT, Wang ZJ, Handa CL, Xu J (2017) Effects of ultrasound pre-treatment on the structure of beta-conglycinin and glycinin and the antioxidant activity of their hydrolysates. Food Chem 218:165–172
Liu Y, Kitts DD (2011) Confirmation that the Maillard reaction is the principle contributor to the antioxidant capacity of coffee brews. Food Res Int 44(8):2418–2424
Haskard CA, Li-Chan ECY (1998) Hydrophobicity of bovine serum albumin and ovalbumin determined using uncharged (PRODAN) and anionic (ANS-) fluorescent probes. J Age Food Chem 46(7):2671–2677
Cattaneo F, Sayago JE, Alberto MR (2014) Anti-inflammatory and antioxidant activities, functional properties and mutagenicity studies of protein and protein hydrolysate obtained from Prosopis alba seed flour. Food Chem 161:391–399
Pearce KN, Kinsella JE (1978) Emulsifying properties of proteins: evaluation of a turbidimetric technique. J Agric Food Chem 26(3):716–723
Dinis TC, Madeira VM, Almeida LM (1994) Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 315:161–169
Oyaizu M (1988) Antioxidative activities of browning products of glucosamine fractionated by organic solvent and thin-layer chromatography. J Jpn Soc Food Sci 35(11):771–775
Amarowicz R, Naczk M, Shahidi F (2000) Antioxidant activity of various fractions of non-tannin phenolics of canola hulls. J Agric Food Chem 48:2755–2759
Delphine L, Claude I, Berger C, Éric V, Laurent D, Fabienne G (2008) Kinetic study on the Maillard reaction. Consideration of sugar reactivity. Food Chem 111(4):1032–1042
Dickinson E, Euston SR (1991) Stability of food emulsions containing both protein and polysaccharide. In: Dickinson E (ed) Food polymers, gels and colloids. The Royal Society of Chemistry, London, pp 132–146
Morales FJ, Jimenez-Perez S (2001) Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. Food Chem 72:119–125
Li CH, Xue Q, Wang X (2014) Comparative studies on the physicochemical properties of peanut protein isolate–polysaccharide conjugates prepared by ultrasonic treatment or classical heating. Food Res Int 57:1–7
Mu L, Zhao M, Yang B, Zhao H, Cui C (2010) Effect of ultrasonic treatment on the graft reaction between soy protein isolate and gum acacia and on the physicochemical properties of conjugates. J Agric Food Chem 58(7):4494–4499
Martínez KD, Sanchez CC, Ruíz-Henestrosa VP, Rodríguez Patino JM, Pilosof AM (2007) Effect of limited hydrolysis of soy protein on the interactions with polysaccharides at the air–water interface. Food Hydrocoll 21:813–822
Liu J, Ru Q, Ding Y (2012) Glycation a promising method for food protein modification: physicochemical properties and structure, a review. Food Res Int 49(1):170–183
Tang CH, Sun X (2011) Modulation of physicochemical and conformational properties of kidney bean vicilin (phaseolin) by glycation with glucose: implications for structure–function relationships of legume vicilind. J Agric Food Chem 59(18):10114–10123
Xue F, li C, Zhu X, Wang L, Pan S (2013) Comparative studies on the physicochemical properties of soy protein isolate–maltodextrin and soy protein isolate–gum acacia conjugate prepared through Maillard reaction. Food Res Int 51(2):490–495
Sheng L, Su P, Han K, Chen J (2017) Synthesis and structural characterization of lysozyme–pullulan conjugates obtained by the Maillard reaction. Food Hydrocoll 71:1–7
Liu Q, Geng R, Zhao J, Chen Q, Kong B (2015) Structural and gen textural properties of soy protein isolate when subjected to extreme acid pH-shifting and mild heating processes. J Agric Food Chem 63(19):4853–4861
Nikolaidis A, Andreadis M, Moschakis T (2017) Effect of heat, pH, ultrasonication and ethanol on the denaturation of whey protein isolate using a newly developed approach in the analysis of difference-UV spectra. Food Chem 232:425–433
Li W, Zhao H, He Z, Zeng M, Qin F (2016) Modification of soy protein hydrolysates by Maillard reaction: effects of carbohydrate chain length on structural and interfacial properties. Colloids Surf B Biointerfaces 138:70–77
Achouri A, Boye JI, Belanger D, Chiron T (2010) Functional and molecular properties of calcium precipitated soy glycinin and the effect of glycation with κ-carrageenan. Food Res Int 43(5):1494–1504
Gasymov OK, Glasgow BJ (2007) ANS fluorescence: potential to augment the identification of the external binding sites of proteins. Biochim Biophys Acta 1774(3):403–411
Liu Q, Kong BH, Xiong YL, Xia XF (2010) Antioxidant activity and functional properties of porcine plasma protein hydrolysate as influenced by the degree of hydrolysis. Food Chem 118(2):403–410
Li B, Bao Z, Xu W, Chi Y (2014) Influence of glycation extent on the physicochemical and gelling properties of soybean β-conglycinin. Eur Food Res Technol 240(2):399–411
Wang ZJ, Han FF, Sui B, Yang Y, Zhang H (2016) Effect of ultrasound treatment on the wet heating Maillard reaction between mung bean [Vigna radiate (L.)] protein isolates and glucose and on structural and physico-chemical properties of conjugates. J Sci Food Agric 96(5):1532–1540
Parker A, Gunning PA, Ng K, Robins MM (1995) How does xanthan stabilise salad dressing. Food Hydrocoll 9(4):333–342
Kong BH, Xiong YL (2006) Antioxidant activity of zein hydrolysates in a liposome system and the possible mode of action. J Agric Food Chem 54(16):6059–6068
Fan JF, Zhang YY, Tan S, Zhou M (2006) Improving functional properties of soy protein hydrolysate by conjugation with curdlan. J Food Sci 71(5):C285–C291
Gu F, Kim JM, Hayat K, Xia S, Feng B (2009) Characteristics and antioxidant activity of ultrafiltrated Maillard reaction products from a casein–glucose model system. Food Chem 117(1):48–54
Guerard F, Sumaya-Martinez MT (2003) Antioxidant effects of protein hydrolysates in the reaction with glucose. J Am Oil Chem Soc 80(5):467–470
Kim JS, Lee YS (2009) Antioxidant activity of Maillard reaction products derived from aqueous glucose/glycine, diglycine, and triglycine model systems as a function of heating time. Food Chem 116(1):227–232
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 31301600), the Natural Science Foundation of Heilongjiang Province (no. LC2017010), Science and Technology Research Project of Education Department of Heilongjiang Province (no. 12541008) and Postdoctoral Project in Heilongjiang Province (no. LBH-Q16012), Academic Backbone Project of Northeast Agricultural University (no. 17XG02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Rights and permissions
About this article
Cite this article
Xu, J., Han, D., Chen, Z. et al. Effect of glucose glycosylation following limited enzymatic hydrolysis on functional and conformational properties of black bean protein isolate. Eur Food Res Technol 244, 1111–1120 (2018). https://doi.org/10.1007/s00217-018-3032-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3032-5