Abstract
Cultivated mushroom species are becoming an increasingly consumed commodity owing to their nutritional value and potential biological activities. There is a strict necessity to conduct a thorough screening of their chemical composition to ensure the quality and safety of final food products. The present study analyzed the content of 67 macro- and microelements and detected 36 of them (macroelements: Ca, K, Mg, Na, P; micronutrients: Cu, Fe, Mn, Mo, Zn; toxic metals: Ag, Al, Cd, Ni and Pb; metalloids: As, B, Ge, Te; platinum group elements (PGEs): Os, Pt, Rh; rare earth elements (REES): Gd, Ho, La, Nd, Pr; other elements: Ba, Bi, Ga, In, Sr, Ti, U, V, Zr) in 12 mushroom species (Agrocybe cylindracea, Auricularia polytricha, Clitocybe maxima, Coprinus comatus, Flammulina velutipes, Grifola frondosa, Hericium erinaceus, Laetiporus sulphureus, Pholiota nameko, Stropharia rugosoannulata, Trametes versicolor, Tremella fuciformis) obtained between 2008 and 2016 from the Polish market but originating from both Poland (small scale local production) and China (available in selected oriental or internet shops only). Elemental composition was highly species-specific and did not follow a taxonomical pattern on the family level. As revealed, G. frondosa contained high concentrations of minerals (Ca, Cu, Fe, Mg, Mn, P and Zn). Most of the studied mushrooms were characterized by a relatively high level of PGEs. Serious food contaminants such as Al, As, Cd and Pb were within safety limits set by the FAO/WHO. Additionally, the study presented Andrews curves as a convenient tool to analyze trends on multidimensional data on chemical composition.
Similar content being viewed by others
Introduction
Edible mushrooms have been valued for centuries for their sensory characteristics and culinary suitability and are well recognized for their nutritional and health benefits. Both fresh and dried mushrooms are a good source of polysaccharides (37–48%), proteins (20–25%), fiber (13–24%), vitamins (e.g., B1, B2, B3, B7, C) and minerals (e.g., K, P, Na, Ca, Mg) while being low in fat content (4–5%) and caloric value [1, 2]. They also contain a number of secondary metabolites, such as phenolic compounds, polyketides, terpenes and steroids which possess various properties beneficial for health [3–5]. Specialty mushrooms have been recognized for their anti-bacterial (e.g., Lentinus edodes), and anti-viral activities (e.g., Agrocybe aegerita and Hypsizigus mamoreus), immune-modulating and anti-tumor properties (e.g., Agaricus blazei, Cordyceps sinensis, Grifola frondosa, Ganoderma lucidum, and Trametes versicolor), and have also been documented as functional foods [6–10].
Globally, several hundred wild mushroom species are recognized as edible although only around twenty are commonly cultivated and consumed [11] and only about 10 species are produced on a commercial scale [12]. The most popular species used for dietary purposes include Agaricus bisporus (white button mushroom), Pleurotus ostreatus (oyster mushroom) and Lentinula edodes (Shiitake mushroom).
Mushrooms are well known to accumulate different elements, including potentially toxic metals and metalloids, and have been proposed as potential bioindicators of environmental pollution [13]. Numerous studies have addressed the chemical composition of wild species, including recently reported values of PGEs and REEs [14–22]. Cultivated specimens have usually been analyzed with respect to the content of some elements in selected mushroom species only [23–26], in general, those important to trade. As experimentally proven, mushrooms cultivated on artificially contaminated substrates can uptake and accumulate health-threatening concentrations of toxic elements such as cadmium, lead [27, 28], mercury [28], silver [29] or arsenic [30]. This highlights the need to perform multi-elemental investigations of cultivated mushrooms available commercially as foodstuffs (Table 1).
Nevertheless, few such “market surveys” have been reported, and those that have been performed to date were limited to the analyses of only a few elements in mushrooms collected from 1 year [23]. As far as the European market is concerned, the only multi-elemental and long-term investigations were conducted for mushroom species of the genus Pleurotus cultivated in Poland [46]. The study described inter-specific differences in element accumulation and concluded that levels of most toxic elements were low, apart from Pb and Nd contents, which in particular cases were of some concern [46]. Pleurotus mushrooms are, after Agaricus sp., the most widely cultivated mushroom species in Poland, with a significant share in global production. In 2015 the production of Pleurotus was over 20 thousand tons and Agaricus about 300 thousand tons. More than 80% of these mushrooms were exported to European Union countries. However, there are some less popular mushroom species which are occasionally available in trade in Poland which have never been characterized in respect to their chemical composition (Table 1).
The present study was undertaken to explore the multi-elemental composition of twelve less popular mushroom species available on the Polish market over an 8-year period (2008–2016) using an inductively coupled plasma optical emission spectrometer (ICP-OES). To the best of our knowledge, the results of this study offer, to date, the most comprehensive insight into the observed elemental contents of the studied species.
Materials and methods
Experimental material
Collected mushroom species were purchased between 2008 and 2016 from the Polish market. Some species originated from China and were distributed in some oriental or internet shops only. The fruiting bodies of three species were obtained from small scale local production in Poland. Their characteristics and the substrates used in cultivation are presented in Table 2. With the exception of C. maxima (from 2010 and 2014) and L. sulphuteus (from 2011, 2013 and 2014), mushrooms were purchased in varying quantities, from relatively few to several fruiting bodies of each species growing in each of the 9 years (about 100 g of fresh or 20 g of dry matter per year). Based on these materials, 3 initial samples were prepared (each of 5 g). For a comparison of mushroom species during the 9 years, although not between particular years, initial samples from all 9 years of the experiment were summed (45 g = 5 g × 9 years). In the case of mushrooms where fruit bodies were not available in all the years of the research, the missing initial samples were prepared based on the rest of the collected bodies. In this way we obtained 36 analytical samples (12 species × 3 initial samples).
Preparation of fruit bodies for element analysis
All collected fruit bodies were dried at 50 ± 2 °C for 30 h and at 80 ± 1 °C for 12 h in an electric oven (SLW 53 STD, Pol-Eko, Wodzisław Śląski, Poland) to dry matter. The 3 initial samples were composed of 5 g of particular mushroom species collected in each year with the above mentioned exceptions. All materials were ground with a laboratory Cutting Mill SM 200 (Retsch GmbH, Haan, Germany) to a powder fraction. Each of the three initial samples collected in all the years was summed to one sample (45 g) and homogenized using a B-400 homogenizator (Buchi Labortechnik AG, Switzerland). This allowed 3 analytical samples per one mushroom species to be obtained (36 samples total).
Accurately weighed 0.3000 ± 0.0001 g of a dry sample of each mushroom species was ultrasonically extracted using phosphoric acid of 1 mol L−1 (Honeywell, USA). After extraction all samples were filtered and diluted with ultrapure water to a final volume of 15.0 mL. Each of the samples was analyzed in triplicate using the whole sample preparation procedure.
Analytical methods
Element determination was conducted using the inductively coupled plasma with optical emission detection model 5100 ICP-OES (Agilent, USA). Evaluation of the content of 67 elements was performed according to the following: for multi-elemental determination common conditions were used: Radio Frequency (RF) power 1.2 kW, nebulizer gas flow 0.7 L min−1, auxiliary gas flow 1.0 L min−1, plasma gas flow 12.0 L min−1, viewing height for radial plasma observation 8 mm, detector Charge Coupled Device (CCD) temperature −40 °C, signal accusation time 5 s for 3 replicates. The detection limits were found at the level of 0.01 mg kg−1 dry weight (DW) for all elements determined (as 3-sigma criteria). The uncertainty for the total analytical procedure (including sample preparation) was at the level of 20%. Traceability was checked using reference materials CRM S-1—loess soil; CRM NCSDC (73349)—bush branches and leaves; CRM 2709—soil; CRM 405—estuarine sediments; CRM 667—estuarine sediments and a recovery (80–120%) was acceptable for most of the elements determined. For uncertified elements recovery was defined using in the standard addition method.
Statistical analysis
Estimation of the content of selected elements (dependent variable) in fruiting bodies of mushroom species (independent variable) was carried out. The mean of element content in particular mushrooms was compared. One-way analysis ANOVA with the F-Fisher test (α = 0.05) was used to verify the general hypothesis with respect to the equality of mean content of particular elements in the analyzed mushroom species. In case the null hypothesis was rejected, the Tukey test for multiple comparisons was applied to divide the studied mushroom species into homogenous groups (α = 0.05) [47].
Andrew curves were applied to present differences in elemental composition among mushrooms. This data visualization allowed the selection of those species in which the content of all elements jointly was relatively lower or higher than in others [48, 49].
Principal Component Analysis (PCA) was used to illustrate the relationships between independent variables (content of elements) for the tested mushroom species. The initial population of variables (mean content of particular elements) X 1, X 2, …, X p , where p = 1, 2, …, 36 was transformed into the population of principal components (Z 1, Z 2, …., Z p). Correlation coefficients between particular variables and components was calculated and based on these results the relationship between the element content of the analyzed mushroom species was interpreted. Moreover, the similarities and differences in the element content of the analyzed mushroom species were determined [50].
To group the tested mushroom species according to similarities in the content of all elements jointly, Hierarchical Cluster Dendrograms were applied. Homogenous groups were created by the ward.D2 agglomeration method (hclust {stats}) with Euclidean Distance.
Results
A total of 36 of 67 of analyzed elements (Ag, Al, As, B, Ba, Bi, Ca, Cd, Cu, Fe, Ga, Gd, Ge, Ho, In, K, La, Mg, Mn, Mo, Na, Nd, Ni, Os, P, Pb, Pr, Pt, Rh, Sr, Te, Ti, U, V, Zn and Zr) were identified above the limit of detection for each investigated mushroom species. Therefore, the comparisons and relationships presented in the following sub-sections were elaborated on this group of elements.
Differences between mushroom species respecting particular element content
Characteristics of the similarities and differences between 12 cultivated mushroom species respecting the accumulation of the thirty-six elements are presented in Table 3.
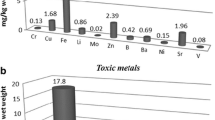
Fruiting bodies of G. frondosa exhibited the highest content (mg kg−1 dm) of Ba (2.5), Ca (2481), Cd (1.9), Fe (214), Ge (1.3), Mg (1453), Mn (32), Mo (0.57), Ni (0.65), P (26,910), Pt (6.0), Rh (0.30), Sr (8.8) and Zn (246) but the lowest concentration of Pb (0.38). L. sulphureus, in turn, was characterized by the lowest levels of Cu (5.0), K (16,023), Mg (481), Mn (6.1), P (10,554), Te (0.37) and Zn (51) but the highest content of Al (23), Pb (1.8), Ti (0.39) and V (0.04). The highest content of Ag (0.40) and Os (0.20) but the lowest content of Ca (155), La (<0.01), Na (41) and Sr (317) was observed in A. cylindracea. The highest concentrations of B (57) and Na (463) were observed for A. polytricha. As (1.2) and K (40,583) in T. fuciformis, A. polytricha and T. fuciformis were the next species to reveal a higher content of two elements only (B (56), Na (463) and As (1.2), K (40,583), respectively) and the lowest content of four other elements (Cd (0.04), Gd (<0.01), Os (<0.01), Zr (0.01) and Mo (0.01), Na (39), Ti (0.04), V (<0.01), respectively. C. comatus contained the highest content of Cu (44) and Pr (1.7) and the lowest, trace amounts of As (<0.01), Bi (<0.01), Ga (0.08), Gd (<0.01) and Ho (<0.01). Similar characteristics were found for F. velutipes and H. erinaceus, where the lowest content of Ga (0.33), Ho (0.30), Te (5.8) and Bi (1.6), In (7.5) and Nd (0.45), were observed in these two, respectively. It is worth underlining that these species were simultaneously characterized by a lower content of Ag (0.04), Ge (0.06), Mo (<0.01), Nd (0.11), Ni (<0.01), Rh (<0.01) and Al (2.9), Bi (1.6), P (10,277), Pr (0.02), U (0.204) and V (<0.01), respectively. The lowest diversity in the analyzed element contents was found in P. nameko and T. versicolor. The highest content of Gd (0.05) and La (0.13) with the lowest content of Mo (<0.01) and As (<0.01), was recorded in both these mushroom species. S. rugosoannulata revealed the lowest contents of B (<0.01), Ba (0.48), Gd (<0.01), In (1.5), Os (<0.01), Rh (<0.01) and V (<0.01). Additionally, it was characterized by a medium content of the remaining of the elements.
Principal Component Analysis explained 27.07% + 15.42% = 42.49% of total variability meaning that 36 elements for 12 mushroom species were well transformed into a 2 dimensional system for graphical presentation of the obtained results.
Some of the presented relationships indicate a generally higher content of the analyzed elements in G. frondosa, F. velutipes and C. comatus (Fig. 1a). Most of the variability (nearly 100%) for G. frondosa was explained with the first principal component. In the case of F. velutipes and C. comatus, almost the whole variability was explained by the second variable component.
T. fuciformis was localized in the center of the coordinate system thereby indicating that elemental content of this species was more similar to the multidimensional mean value than any other mushroom species investigated in the present study (Fig. 1a). It is difficult to show that such a localization of T. fuciformis among the tested mushroom species will be the same each time. To confirm these relationships, it will be necessary to conduct further studies because when considering the effective transport of 36 elements it is essential not to ignore the important role of the interactions between elements and, in particular, their influence on the nutritional elements in mushroom bodies.
Other mushroom species were placed above the ring, which suggests a generally higher or lower content of all elements jointly than in other groups of similar species present inside the ellipsoid (Fig. 1b). Differences between the three groups of elements marked with green rings were indicated. According to data presented in Fig. 1b, the content of Pb was adverse to B, Pt, Pr, Mo, Sr, Ca, Cd, Zn, Mg, Mn, Fe and P content.
Simultaneously, content of Zr, Os, Bi, As, Ga, Te, U and Gd in mushrooms was adverse to Ag, Nd, Al, Ti, Cu and Ni content. It is worth noting that content of Zr, Os, Bi, As, Ga and Te was independent of B, Pt, Pr, Mo, Sr, Ca, Cd, Zn, Mg, Mn, Fe, P and U content. The relationships between element contents described in our study are probably an effect of their differences in accumulation and toxicity. Pb is a heavy metal usually characterized by limited transport, whereas especially numerous elements contained within the group placed on the right side are more mobile and in some cases, such as nutritional elements, essential for the proper functioning of mushrooms. Unfortunately, we have no data on the detailed characteristics of the substrate used in production, thus another possible cause of such relationships could also be the concentration of Pb in substrates and their pH and conductivity.
Differences between mushroom species respecting all elements jointly
To compare the analyzed mushroom species with respect to the content of all 36 elements jointly, a cluster analysis was applied which allowed 6 groups of mushrooms to be distinguished (Fig. 2). In the first three groups, only C. maxima, G. frondosa and S. rugosoannulata, were present.
The two latter mushroom species were more similar to each other than C. maxima. In the fourth group, the following species were included: A. cylindracea, L. sulphureus, P. nameko and T. versicolor. Within this group differences were found in element contents between the first two and the other mushroom species. In the fifth group, A. polytricha and C. comatus were found to be similar while some differences were noted respecting F. velutipes. T. fuciformis and H. erinaceus are included in the sixth and last group.
The differences between the investigated species were also demonstrated using Andrews curves calculated for all the analyzed fruiting bodies collected from each species (Fig. 3).
As illustrated, the curve drawn for T. fuciformis (light blue) had the highest amplitude, indicating that the elemental composition of this species differed from other mushrooms. This was also confirmed by plotting Andrews curves for 3 randomly selected observations (elements) for each species—a similar amplitude of curves was observed (Fig. 3b).
Figure 3a presents the mean values calculated for all analyzed fruiting bodies for particular mushroom species. T. fuciformis, the mushroom species located in the center of PCA ring (Fig. 1a), in this case is presented as a blue curve with the highest amplitude. This suggests that this species differs from the others in terms of elemental content. It is interesting to note that for 3 randomly selected observations for particular mushroom species (Fig. 3b), similar tendencies (amplitude of curves) were observed thereby confirming the observations presented in Table 3.
Discussion
The present study demonstrates the large set of data of multi-elemental compositions of mushroom species that are available on the Polish market. The studied species are less popular than Pleurotus ostreatus, Agaricus bisporus or Lentinula edodes but their popularity, distribution and sale may potentially rise in the near future. This can be expected due to the growing interest in the use of mushrooms in medicinal nutrition therapies and diet [51]. This considered, it is of great importance to screen and compare the chemical composition of mushrooms. However, in the literature, there is a great deal of data from investigations that tend to focus on wild-growing species, while the majority of cultivated specimens are usually studied for the content of some elements in selected mushroom species [23–26]. Contrary to the majority of studies, the present research demonstrates the results of 36 elements in 12 cultivated species whose fruiting bodies were collected over 8 years, and provides some informational values on content for their nutritional value and contamination levels.
As shown using different statistical approaches, the investigated species differ in the content of certain elements including minerals, and potentially toxic metals and metalloids. Particularly useful visualization of multidimensional data was acquired by plotting Andrews curves in which outliers appear as a single curve that differs from the rest in its amplitude. Although such an approach to the illustration of data has been successfully applied in biomedical and pharmacological sciences [52, 53], we are unaware of any similar application in food chemistry. If one considers that chemical screenings often require the collection of large data sets, Andrews curves may be helpful in their further interpretation, and selection of those items which clearly differ from others.
The cluster analysis revealed that there is no particular pattern as regards elemental composition related to taxonomic position (family) of the studied mushrooms or their ecology (saprotrophic or parasitic). Similarly, the PCA analysis did not demonstrate any existence of such a pattern. Therefore, the observed differences can be attributed to: (1) different composition of substrate used for their cultivation, and/or (2) inter-specific differences in mycelia uptake [54].
Compared to other species, the fruiting bodies G. frondosa were revealed to be the greater source of Ca, Cu, Fe, Mg, Mn, P or Zn [13, 55, 56] and lower in the case of, e.g., sum of REEs [22]. All of these elements play a pivotal role in various physiological processes while their deficiencies have been implicated in the etiology of number of disorders [57]. Fe and Zn deficiency in particular appears to be a global health issue, affecting the populations of both developed and undeveloped countries [58]. Our finding highlights the nutritional value of G. frondosa which was previously shown to contain high concentrations of starch and total dietary fiber [59]. Although the bioavailability of Ca, Cu, Fe, Mg, Mn, P and Zn in G. frondosa remains to be studied more fully, this species would appear to be a promising candidate for the dietary source of different minerals in the human diet.
There is an increasing interest in the content of REEs in different environmental, biological and food samples [60]. Over the last decade, there has been a significant increase in their use, mostly in the industrial sector, and their subsequent release to the environment is being observed. Despite this, toxicological investigations on REE-related adverse effects to human health are still scarce, and not much is known as to their presence in mushrooms. Recently, some studies have reported the presence of REEs in wild-edible species [22], and our previous studies have described their content in cultivated mushrooms from the Pleurotus genus [46]. In the present study, each sample contained detectable levels of five REEs whose mean content generally decreased in the following order: Pr > Nd > Ho > La > Gd. Particularly high Pr content, exceeding 1 mg kg−1 was found in C. comatus, T. fuciformis, F. velutipes, G. frondosa, L. sulphureus and T. versicolor. So far the only existing regulation, implemented in China, sets a maximum content of total REEs in food at 0.7 mg kg−1 fresh weight [61], which accounts for 7 mg kg−1 dw (if one considers the usual level of 10% water content). This maximum was not exceeded by any studied species in the present study. Nevertheless, the present study draws attention to the fact that in any further legislation concerning this elemental group mushrooms should be considered as a potentially important dietary source of REEs, particularly Pr and Nd.
The present study also identified some PGEs in cultivated mushrooms; namely Pt, Rh and Os. It has been shown that environmental concentrations of PGEs are steadily increasing due to their use in automobile catalytic converters. Chronic, sub-clinical exposure to these elements may pose a health risk, particularly in vulnerable groups such as children. It has been suggested that nearly half of human PGE exposure occurs through diet [62, 63]. In spite of this, information respecting PGEs in different foodstuffs, including mushrooms, is scarce. Our previous screening of 20 wild-edible species (including G. frondosa and F. velutipes, whose specimen from cultivation were tested in the present study) revealed generally low content of PGEs which for Pt, Rh and Os was in range of 0.01–0.03 mg kg−1 dw [22]. The present study observed levels higher by orders of magnitude but in line with our previous findings in different cultivated mushrooms from the Pleurotus genus [46]. The general mean content of PGEs in the present study decreased in the following order: Pt > Rh > Os. Particularly high Pt concentrations (exceeding 5 mg kg−1 dw) were found for G. frondosa, C. comatus, F. velutipes and S. rugosoannulata. All in all, this highlights that cultivated mushrooms may be an important dietary source of PGEs; the reasons behind it are yet to be elucidated. As previously shown, the increased concentrations of various pollutants in fruiting bodies of cultivated mushrooms can be associated by their increased content in overgrown substrate [23]. Therefore, it would be highly advisable to screen PGE content in cultivation substrates as their origin may be distinctively different. Typical substrates which are used include wheat straw, gypsum, oak and beech sawdust, or chicken manure [64, 65]. All of the above can potentially be contaminated, particularly if collected from polluted areas.
As previously demonstrated experimentally, As presence in substrate can result in high levels of accumulation of this toxic metalloid in cultivated mushrooms. This strongly advocates the necessity to control As levels in different species which are available in trade to ensure their safety for human health. The present study found distinctively different mean As levels for the investigated mushrooms species ranging from 0.001 mg kg−1 dm (C. comatus, H. erinaceus and T. versicolor) to over 1.0 mg kg−1 dm (F. velutipes and T. fuciformis). Similar or higher levels of As have been found in commercial mushrooms from cultivation [23, 46], as well as other food products and dietary supplements [66]. As toxicity largely depends on its form (with inorganic compounds being more toxic than organic) and the provisional tolerable weekly intake (PTWI) of total As was set at 15 µg kg−1 body weight (later withdrawn with no new PTWI set) [67, 68].
Maximum allowance levels of Cd and Pb in mushrooms (set only for Agaricus bisporus, Pleurotus ostreatus and Lentinula edodes) set by the European Commission are 0.2 and 0.3 mg kg−1 dm, respectively which accounts for 2.0 and 3.0 mg kg−1 dw, respectively, if one considers the usual level of 10% water content. Mean concentrations obtained for the mushrooms investigated in the present study did not exceed this. If one will further consider that the bioaccessibility of toxic elements from the investigated mushrooms in the human gastrointestinal tract can be reduced by cooking treatments such as boiling or microwaving with water [69], consumption of the investigated species would not contribute significantly to Cd or Pb exposure.
Finally, some mushrooms examined in the present study contained relatively high Al content, exceeding 20 mg kg−1 dm for L. sulphureus. It should, however, be stressed that Al, which is the most ubiquitous metal in the earth’s crust and has no known biological function, is poorly absorbed in the gastrointestinal tract (in the range of a 0.1–1.0% oral dose), and in healthy subjects almost all the absorbed Al is excreted readily from the body [70]. The provisional tolerable weekly intake (PTWI) for Al is 2 mg kg−1 bodyweight [71]. Considering an average single serving is 300 g of fresh mushrooms, i.e., about 30 g of dry matter (as a 10% level is used for calculations with an unknown factual dm level), the content determined in the investigated mushrooms would contribute insignificantly to PTWI for an adult weighing 60 kg.
Conclusions
The present study screened the elemental content of fruiting bodies of 12 mushroom species. The studied mushrooms revealed differences in chemical content as shown using different statistical tools, including Andrews curves which appear to be a convenient method for illustrating multidimensional data on food composition. As revealed, G. frondosa appeared to be a highly nutritional mushroom with an exceptional Ca content. Relatively high levels of PGEs were found in the studied species. Further studies are yet to be conducted to elucidate the strikingly high content of PGEs in some of the mushrooms.
References
Alam N, Amin R, Khan A, Ara I, Shim MJ, Lee MW, Lee TS (2008) Nutritional analysis of cultivated mushrooms in Bangladesh—Pleurotus ostreatus, Pleurotus sajor-caju, Pleurotus florida and Calocybe indica. Mycobiology 36:228–232
Chang S-T, Miles PG (2004) Cultivation, nutritional value, medicinal effect, and environmental impact, 2nd edn. CRC Press, Boca Raton
Cheung LM, Cheung PCK, Ooi VEC (2003) Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem 81:249–255
Davoli P, Mucci A, Schenetti L, Weber RWS (2005) Laetiporic acids, a family of non-carotenoid polyene pigments from fruit-bodies and liquid cultures of Laetiporus sulphureus (Polyporales, Fungi). Phytochemistry 66:817–823
Turkoglu A, Duru ME, Mercan N, Kivrak I, Gezer K (2007) Antioxidant and antimicrobial activities of Laetiporus sulphureus (Bull.) Murrill. Food Chem 101:267–273
Tsai SY, Huang SJ, Lo SH, Wu TP, Lian PY, Mau JL (2009) Flavour components and antioxidant properties of several cultivated mushrooms. Food Chem 113:578–584
Vaz JA, Barros L, Martins A, Santos-Buelga C, Vasconcelos MH, Ferreira ICFR (2011) Chemical composition of wild edible mushrooms and antioxidant properties of their water soluble polysaccharidic and ethanolic fractions. Food Chem 126:610–616
Alves MJ, Ferreira IC, Dias J, Teixeira V, Martins A, Pintado M (2012) A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Med 78:1707–1718
Guggenheim AG, Wright KM, Zwickey HL (2014) Immune modulation from five major mushrooms: application to integrative oncology. Integr Med 13:32–44
Jie-Jen L, Joen-Rong S, Woan-Ching J, Chien-Liang L (2014) Water extracts of dietary mushrooms, Agrocybe aegerita and Hypsizigus mamoreus, inhibit antigen expression of human hepatitis B virus. J Med Plants Res 8:246–252
Kalač P (2013) A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J Sci Food Agr 93:209–218
Reis FS, Barros L, Sousa MJ, Martins A, Ferreira ICFR (2014) Analytical methods applied to the chemical characterization and antioxidant properties of three wild edible mushroom species from northeastern Portugal. Food Anal Methods 7:645–652
Mleczek M, Niedzielski P, Kalač P, Budka A, Siwulski M, Gąsecka M, Rzymski P, Magdziak Z, Sobieralski K (2016) Multielemental analysis of 20 mushroom species growing near a heavily trafficked road in Poland. Environ Sci Pollut Res Int 23:16280–16295
Falandysz J, Sapkota A, Mędyk M, Feng X (2017) Rare earth elements in parasol mushroom Macrolepiota procera. Food Chem 221:24–28
Kalač P (2010) A review of trace element concentrations in edible mushrooms. Food Chem 69:273–281
Tüzen M (2003) determination of heavy metals in soil, mushroom and plant samples by atomic absorption spectrometry. Microchem J 74:289–297
Doğan HH, Sanda MA, Uyanöz R, Oztürk C, Cetin U (2006) Contents of metals in some wild mushrooms: its impact in human health. Biol Trace Elem Res 110:79–94
Kalač P (2009) Chemical composition and nutritional value of European species of wild growing mushrooms: a review. Food Chem 113:9–16
Gençcelep H, Uzun Y, Tunçtürk Y, Demirel K (2009) Determination of mineral contents of wild-grown edible mushrooms. Food Chem 113:1033–1036
Säumel I, Schlecht MT, Ina S (2015) Wild growing mushrooms for the Edible City? Cadmium and lead content in edible mushrooms harvested within the urban agglomeration of Berlin, Germany. Environ Pollut 204:298–305
Cordeiro F, Llorente-Mirandes T, López-Sánchez JF, Rubio R, Sánchez Agullo A, Raber G, de la Calle MB (2015) Determination of total cadmium, lead, arsenic, mercury and inorganic arsenic in mushrooms: outcome of IMEP-116 and IMEP-39. Food Addit Contam Part A 32:54–67
Mleczek M, Niedzielski P, Kalač P, Siwulski M, Rzymski P, Gąsecka M (2016) Levels of platinum group elements and rare-earth elements in wild mushroom species growing in Poland. Food Addit Contam Part A 33:86–94
Huang Q, Jia Y, Wan Y, Li H, Jiang R (2015) Market survey and risk assessment for trace metals in edible fungi and the substrate role in accumulation of heavy metals. J Food Sci 80:1612–1618
Koyyalamudi SR, Jeong S, Manavalan S, Vysetti B, Pang G (2013) Micronutrient mineral content of the fruiting bodies of Australian cultivated Agaricus bisporus white button mushrooms. J Food Comp Anal 31:109–114
Vetter J, Hajdú J, Györfi J, Maszlavér P (2005) Mineral composition of the cultivated mushrooms Agaricus bisporus, Pleurotus ostreatus and Lentinula edodes. Acta Alim 34:441–451
Vetter J (2003) Chemical composition of fresh and conserved Agaricus bisporus mushroom. Eur Food Res Technol 217:10–12
Enke M, Roschig M, Matschiner H, Achtzehn MK (1979) Uptake of lead, cadmium and mercury by cultivated mushrooms. Nahrung 23:731–737
Rzymski P, Mleczek M, Siwulski M, Gąsecka M, Niedzielski P (2016) The risk of high mercury accumulation in edible mushrooms cultivated on contaminated substrates. J Food Comp Anal 51:55–60
Falandysz J, Bona H, Danisiewicz D (1994) Silver uptake by Agaricus bisporus from an artificially enriched substrate. Z Lebensm Unters Forsch 199:225–228
Mleczek M, Niedzielski P, Siwulski M, Rzymski P, Gąsecka M, Goliński P, Kozak L (2016) Importance of low substrate As content in mushroom cultivation and safety of final food product. Eur Food Res Technol 242:1–8
Liu Y-T, Sun J, Luo Z-Y, Rao S-Q, Su Y-J, Xu R-R, Yang Y-J (2012) Chemical composition of five wild edible mushrooms collected from Southwest China and their antihyperglycemic and antioxidant activity. Food Chem Toxicol 50:1238–1244
Cocchi L, Vescovi L, Petrini LE, Petrini O (2006) Heavy metals in edible mushrooms in Italy. Food Chem 98:277–284
Zhu F, Qu L, Fan W, Qiao M, Hao H, Wang X (2011) Assessment of heavy metals in some wild edible mushrooms collected from Yunnan Province, China. Environ Monit Assess 179:191–199
Campos JA, De Toro JA, Perez De Los Reyes C, Amoros JA, Garcia-Moreno R (2012) Lifestyle influence on the content of copper, zinc and rubidium in wild mushrooms. Appl Environ Soil Sci ID 687160
Michelot D, Siobud E, Dore JC, Viel C, Poirier F (1998) Update on metal content profiles in mushrooms-toxicological implications and tentative approach to the mechanisms of bioaccumulation. Toxicon 36:1997–2012
Dulay RMR, Pascual AHL, Constante RD, Tiniola RC, Areglo JL, Arenas MC, Reyes RG (2015) Growth response and mycoremediation activity of Coprinus comatus on heavy metal contaminated media. Mycosphere 6:1–7
Severoglu Z, Sumer S, Yalcin B, Leblebici Z, Aksoy A (2013) Trace metal levels in edible wild fungi. Int J Environ Sci Technol 10:295–304
Kim JY, Yoo JH, Lee JH, Kim MJ, Kang DW, Ko Hong SM, Im GJ, Kim DH, Jung GB, Kim WI (2012) Monitoring and risk assessment of heavy metals in edible mushrooms. Korean J Environ Agric 31:37–44
Smiderle FR, Carbonero ER, Sassaki GL, Gorin PAJ, Iacomini M (2008) Characterization of a heterogalactan: some nutritional values of the edible mushroom Flammulina velutipes. Food Chem 108:329–333
Smiderle FR, Olsen LM, Carbonero ER, Baggio CH, Freitas CS, Marcon R, Santos ARS, Gorin PAJ, Iacomini M (2008) Anti-inflammatory and analgesic properties in a rodent model of a (1 → 3), (1 → 6)-linked β-glucan isolated from Pleurotus pulmonarius. Eur J Pharmacol 597:86–91
Rodrigues DMF, Freitas AC, Rocha-Santos TAP, Vasconcelos MW, Roriz M, Rodriguez-Alcala LM, Duarte AC (2015) Chemical composition and nutritive value of Pleurotus citrinopileatus var cornucopiae, P. eryngii, P. salmoneo stramineus, Pholiota nameko and Hericium erinaceus. J Food Sci Tech 52:6927–6939
Agafonova SV, Olennikov DN, Borovskii GB, Penzina TA (2007) Chemical composition of fruiting bodies from two strains of Laetiporus sulphureus. Chem Nat Compd 43:569–570
Florczak J, Niedźwiecka E, Wędzisz A (2009) Skład chemiczny i aktywność celulolityczna łuskwiaka nameko – Pholiota nameko. [Chemical composition and the cellulolytic activity of Pholiota nameko]. BromatChem Toksykol 1:65–69 (in Polish)
Manjunathan J, Subbulakshmi N, Shanmugapriya R, Kaviyarasan V (2011) Proximate and mineral composition of four edible mushroom species from South India. Int J Biodivers Conserv 3:386–388
Anno AH, Konan HK, Kouadio JPE, Dué EA, Kouamé LP (2016) Chemical composition and nutritional value of two edible mushrooms from three regions of Côte d’Ivoire. J Basic Appl Res 2:119–125
Siwulski M, Mleczek M, Rzymski P, Budka A, Jasińska A, Niedzielski P, Kalac P, Gąsecka M, Budzyńska S, Mikołajczak P (2017) Screening the multi-element content of Pleurotus mushroom species using inductively coupled plasma optical emission spectrometer (ICP-OES). Food Anal Method 10:487–496
Chambers JM, Freeny AE, Heiberger RM (1992) Analysis of variance; designed experiments. In: Chambers JM, Hastie TJ (eds) Statistical models in S. Wadsworth & Brooks/Cole, Pacific Grove
Andrews DF (1972) Plots of high-dimensional data. Biometrics 28:125–136
Khattree R, Naik DN (2002) Andrews plots for multivariate data: some new suggestions and applications. J Stat Plan Inference 100:411–425
Krzanowski WJ (2000) Principles of multivariate analysis: a user’s perspective. Oxford University Press, Oxford
Kasper-Pakosz R, Pietras M, Łuczaj Ł (2016) Wild and native plants and mushrooms sold in the open-air markets of south-eastern Poland. J Ethnobiol Ethnomed 12:45
Vorster HH, Benade AJ, Barnard HC, Locke MM, Silvis N, Venter CS, Smuts CM, Engelbrecht GP, Marais MP (1992) Egg intake does not change plasma lipoprotein and coagulation profiles. Am J Clin Nutr 55:400–410
El-Masri HA, Kenyon EM (2008) Development of a human physiologically based pharmacokinetic (PBPK) model for inorganic arsenic and its mono- and di-methylated metabolites. J Pharmacokinet Pharmacodyn 35:31–68
Kalač P (2016) Edible mushrooms. Chemical composition and nutritional value. Academic Press/Elsevier, Amsterdam
Shimaoka I, Kodama J, Nishino K, Itokawa Y (1993) Purication of a copper binding peptide from the mushroom Grifola frondosa and its effect on copper absorption. J Nutr Biochem 4:33–38
Wang C, Hou Y (2011) Determination of trace elements in three mushroom samples of basidiomycetes from Shandong, China. Biol Trace Elem Res 142:843–847
Mwiti Kibiti C, Jide Afolayan A (2015) The biochemical role of macro and micro-minerals in the management of diabetes mellitus and its associated complications: a review. Int J Vitam Nutr Res 85:88–103
Bailey RL, West KP Jr, Black RE (2015) The epidemiology of global micronutrient deficiencies. Ann Nutr Metab 66:22–33
Dikeman CL, Bauer LL, Flickinger EA, Fahey GC Jr (2005) Effects of stage of maturity and cooking on the chemical composition of select mushroom varieties. J Agric Food Chem 53:1130–1138
Yang L, Wang X, Nie H, Shao L, Wang G, Liu Y (2016) Residual levels of rare earth elements in freshwater and marine fish and their health risk assessment from Shandong, China. Mar Pollut Bull 107:393–397
Standardization Administration of the People’s Republic of China (SAC). Maximum levels of contaminants in foods; GB 2762-2012
Cabrera-Vique C, Teissedre PL, Cabanis MT, Cabanis JC (1997) Determination of platinum in wine by graphite furnace atomic absorption spectrometry. J AOAC Int 80:57–62
Ek KH, Rauch S, Morrison GM, Lindberg P (2004) Platinum group elements in raptor eggs, faeces, blood, liver and kidney. Sci Total Environ 334–335:149–159
Sánchez C (2010) Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl Microbiol Biotechnol 85:1321–1337
Niedzielski P, Mleczek M, Siwulski M, Gąsecka M, Kozak L, Rissmann I, Mikołajczak P (2014) Efficacy of supplementation of selected medicinal mushrooms with inorganic selenium salts. J Environ Sci Health B 49:929–937
Hedegaard R, Vingborg S (2013) Total and inorganic arsenic in dietary supplements based on herbs, other botanicals and algae—a possible contributor to inorganic arsenic exposure. Anal Bioanal Chem 405:4429–4435
JECFA 658. Arsenic. WHO food additive series 24, 1998
FAO, WHO (2011) Safety evaluation of certain contaminants in food. WHO food additive series 63/FAO JECFA monographs 8. WHO Press, Geneva
Sun L, Liu G, Yang M, Zhuang Y (2012) Bioaccessibility of cadmium in fresh and cooked Agaricus blazei Murill assessed by in vitro biomimetic digestion system. Food Chem Toxicol 50:1729–1733
Taylor GA, Moore PB, Ferrier IN, Tyrer SP, Edwardson JA (1998) Gastrointestinal absorption of aluminium and citrate in man. J Inorg Biochem 69:165–169
JECFA (2012) Safety evaluation of certain food additives/prepared by the seventy fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), WHO food additives series, vol 65
Acknowledgements
Piotr Rzymski is supported by the Foundation for Polish Science within the “Start” Program (091.2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Przemysław Niedzielski declares that he has no conflict of interest. Mirosław Mleczek declares that he has no conflict of interest. Anna Budka declares that he has no conflict of interest. Piotr Rzymski declares that he has no conflict of interest. Marek Siwulski declares that he has no conflict of interest. Agnieszka Jasińska declares that he has no conflict of interest. Monika Gąsecka declares that she has no conflict of interest. Sylwia Budzyńska declares that she has no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Niedzielski, P., Mleczek, M., Budka, A. et al. A screening study of elemental composition in 12 marketable mushroom species accessible in Poland. Eur Food Res Technol 243, 1759–1771 (2017). https://doi.org/10.1007/s00217-017-2881-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-017-2881-7