Abstract
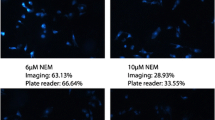
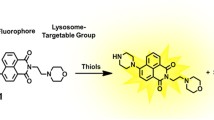
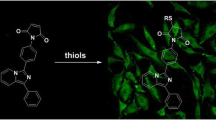
Thiol molecules play a significant role in cellular structures and functions. These molecules are distributed in cells unevenly at the subcellular level. Disturbance of cellular thiols has been associated with various diseases and disorders. Probes that are able to detect subcellular thiol density in live cells are valuable tools in determining thiols’ roles at the subcellular level. Lysosomes are a subcellular organelle involved in the degradation of macromolecules through the action of proteolytic enzymes. The degradation not only serves as a way to dispose of unwanted macromolecules but also a way to regulate a variety of cellular functions such as autophagy, endocytosis, and phagocytosis to maintain cell homeostasis. A probe that can detect lysosomal thiols in live cells will be useful in unveiling the roles of thiols in lysosomes. Currently, limited probes are available to detect lysosomal thiols in live cells. We would like to report 4,4′-{[7,7′-thiobis(benzo[c][1,2,5]oxadiazole-4,4′-sulfonyl)]bis(oxy))bis(naphthalene-2,7-disulfonicacid) (TBONES) as a thiol-specific fluorogenic agent for lysosomal thiol imaging in live cells through fluorescence microscopy. TBONES exhibits no fluorescence and readily reacts with non-protein thiols to form fluorescent thiol adducts with λex = 400 nm and λem = 540 nm. No reaction was observed when TBONES was mixed with compounds containing nucleophilic functional groups other than thiols such as –OH, –NH2, and –COOH. No reaction was observed either when TBONES was mixed with protein thiols. When incubated with cells, TBONES selectively and effectively imaged lysosomal thiols in live cells. Imaging of lysosomal thiols was confirmed by a co-localization experiment with LysoTracker™ Blue DND-22.











Similar content being viewed by others
References
Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. https://doi.org/10.1146/annurev.cb.05.110189.002411.
Winchester BG. Lysosomal membrane proteins. Eur J Paediatr Neurol. 2001;5(Suppl A):11–9.
Mindell JA. Lysosomal acidification mechanisms. Annu Rev Physiol. 2012;74:69–86. https://doi.org/10.1146/annurev-physiol-012110-142317.
Zhu H, Fan J, Xu Q, Li H, Wang J, Gao P, et al. Imaging of lysosomal pH changes with a fluorescent sensor containing a novel lysosome-locating group. Chem Commun (Camb). 2012;48(96):11766–8. https://doi.org/10.1039/c2cc36785h.
Ferguson SM. Neuronal lysosomes. Neurosci Lett. 2019;697:1–9. https://doi.org/10.1016/j.neulet.2018.04.005.
Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat Rev Mol Cell Biol. 2009;10(9):623–35. https://doi.org/10.1038/nrm2745.
Ballabio A. The awesome lysosome. EMBO Mol Med. 2016;8(2):73–6. https://doi.org/10.15252/emmm.201505966.
Appelqvist H, Waster P, Kagedal K, Ollinger K. The lysosome: from waste bag to potential therapeutic target. J Mol Cell Biol. 2013;5(4):214–26. https://doi.org/10.1093/jmcb/mjt022.
Kamat PK, Kyles P, Kalani A, Tyagi N. Hydrogen sulfide ameliorates homocysteine-induced Alzheimer’s disease-like pathology, blood-brain barrier disruption, and synaptic disorder. Mol Neurobiol. 2016;53(4):2451–67. https://doi.org/10.1007/s12035-015-9212-4.
Dielschneider RF, Henson ES, Gibson SB. Lysosomes as oxidative targets for cancer therapy. Oxidative Med Cell Longev. 2017;2017:3749157. https://doi.org/10.1155/2017/3749157.
Reichmann D, Voth W, Jakob U. Maintaining a healthy proteome during oxidative stress. Mol Cell. 2018;69(2):203–13. https://doi.org/10.1016/j.molcel.2017.12.021.
Van Laer K, Hamilton CJ, Messens J. Low-molecular-weight thiols in thiol-disulfide exchange. Antioxid Redox Signal. 2013;18(13):1642–53. https://doi.org/10.1089/ars.2012.4964.
Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43(2):143–81. https://doi.org/10.1080/10408360500523878.
Maher P. The effects of stress and aging on glutathione metabolism. Ageing Res Rev. 2005;4(2):288–314. https://doi.org/10.1016/j.arr.2005.02.005.
McMurray J, Chopra M, Abdullah I, Smith WE, Dargie HJ. Evidence of oxidative stress in chronic heart failure in humans. Eur Heart J. 1993;14(11):1493–8.
Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6. https://doi.org/10.1186/1475-2891-14-6.
Wang W, Rusin O, Xu X, Kim KK, Escobedo JO, Fakayode SO, et al. Detection of homocysteine and cysteine. J Am Chem Soc. 2005;127(45):15949–58. https://doi.org/10.1021/ja054962n.
McBean GJ, Aslan M, Griffiths HR, Torrao RC. Thiol redox homeostasis in neurodegenerative disease. Redox Biol. 2015;5:186–94. https://doi.org/10.1016/j.redox.2015.04.004.
Shahrokhian S. Lead phthalocyanine as a selective carrier for preparation of a cysteine-selective electrode. Anal Chem. 2001;73(24):5972–8.
Maeda H, Matsuno H, Ushida M, Katayama K, Saeki K, Itoh N. 2,4-Dinitrobenzenesulfonyl fluoresceins as fluorescent alternatives to Ellman's reagent in thiol-quantification enzyme assays. Angew Chem Int Ed Eng. 2005;44(19):2922–5. https://doi.org/10.1002/anie.200500114.
Wei W, Liang X, Hu G, Guo Y, Shao S. A highly selective colorimetric probe based on 2,2′,2″-trisindolylmethene for cysteine/homocysteine. Tetrahedron Lett. 2011;52(13):4.
Hurd TR, Prime TA, Harbour ME, Lilley KS, Murphy MP. Detection of reactive oxygen species-sensitive thiol proteins by redox difference gel electrophoresis: implications for mitochondrial redox signaling. J Biol Chem. 2007;282(30):22040–51. https://doi.org/10.1074/jbc.M703591200.
Chen W, Zhao Y, Seefeldt T, Guan X. Determination of thiols and disulfides via HPLC quantification of 5-thio-2-nitrobenzoic acid. J Pharm Biomed Anal. 2008;48(5):1375–80. https://doi.org/10.1016/j.jpba.2008.08.033.
Chen SJ, Chang HT. Nile red-adsorbed gold nanoparticles for selective determination of thiols based on energy transfer and aggregation. Anal Chem. 2004;76(13):3727–34. https://doi.org/10.1021/ac049787s.
Arunachalam B, Phan UT, Geuze HJ, Cresswell P. Enzymatic reduction of disulfide bonds in lysosomes: characterization of a gamma-interferon-inducible lysosomal thiol reductase (GILT). Proc Natl Acad Sci U S A. 2000;97(2):745–50.
Mego JL. Role of thiols, pH and cathepsin D in the lysosomal catabolism of serum albumin. Biochem J. 1984;218(3):775–83.
Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol. 2006;46:215–34. https://doi.org/10.1146/annurev.pharmtox.46.120604.141122.
Rao J, Dragulescu-Andrasi A, Yao H. Fluorescence imaging in vivo: recent advances. Curr Opin Biotechnol. 2007;18(1):17–25. https://doi.org/10.1016/j.copbio.2007.01.003.
Dai CG, Du XJ, Song QH. Acid-activatable Michael-type fluorescent probes for thiols and for labeling lysosomes in live cells. J Organomet Chem. 2015;80(24):12088–99. https://doi.org/10.1021/acs.joc.5b02041.
Kand D, Saha T, Lahiri M, Talukdar P. Lysosome targeting fluorescence probe for imaging intracellular thiols. Org Biomol Chem. 2015;13(30):8163–8. https://doi.org/10.1039/c5ob00889a.
Cao M, Chen H, Chen D, Xu Z, Liu SH, Chen X, et al. Naphthalimide-based fluorescent probe for selectively and specifically detecting glutathione in the lysosomes of living cells. Chem Commun (Camb). 2016;52(4):721–4. https://doi.org/10.1039/c5cc08328a.
Fan J, Han Z, Kang Y, Peng X. A two-photon fluorescent probe for lysosomal thiols in live cells and tissues. Sci Rep. 2016;6:19562. https://doi.org/10.1038/srep19562.
Liang B, Wang B, Ma Q, Xie C, Li X, Wang S. A lysosome-targetable turn-on fluorescent probe for the detection of thiols in living cells based on a 1,8-naphthalimide derivative. Spectrochim Acta A Mol Biomol Spectrosc. 2018;192:67–74. https://doi.org/10.1016/j.saa.2017.10.044.
Chen C, Zhou L, Liu W, Liu W. Coumarinocoumarin-based two-photon fluorescent cysteine biosensor for targeting lysosome. Anal Chem. 2018;90(10):6138–43. https://doi.org/10.1021/acs.analchem.8b00434.
Wang Y, Liu L, Zhou XL, Wu MY. Lysosome-targeted single fluorescence probe for two-channel imaging intracellular SO(2) and biothiols. Molecules. 2019;24(3):618. https://doi.org/10.3390/molecules24030618.
Li Y, Yang Y, Guan X. Benzofurazan sulfides for thiol imaging and quantification in live cells through fluorescence microscopy. Anal Chem. 2012;84(15):6877–83. https://doi.org/10.1021/ac301306s.
Wang S, Yin H, Huang Y, Guan X. Thiol specific and mitochondria selective fluorogenic benzofurazan sulfide for live cell nonprotein thiol imaging and quantification in mitochondria. Anal Chem. 2018;90(13):8170–7. https://doi.org/10.1021/acs.analchem.8b01469.
Yang Y, Guan X. Non-protein thiol imaging and quantification in live cells with a novel benzofurazan sulfide triphenylphosphonium fluorogenic compound. Anal Bioanal Chem. 2017;409(13):3417–27. https://doi.org/10.1007/s00216-017-0285-y.
Yang Y, Guan X. Rapid and thiol-specific high-throughput assay for simultaneous relative quantification of total thiols, protein thiols, and nonprotein thiols in cells. Anal Chem. 2015;87(1):649–55. https://doi.org/10.1021/ac503411p.
Niu LY, Zheng HR, Chen YZ, Wu LZ, Tung CH, Yang QZ. Fluorescent sensors for selective detection of thiols: expanding the intramolecular displacement based mechanism to new chromophores. Analyst. 2014;139(6):1389–95. https://doi.org/10.1039/c3an01849k.
Krise JP. Intracellular delivery and disposition of small molecular weight drugs. In: Binghe Wang LH, Teruna J, editors. Siahaan (ed) drug delivery: principles and applications. 2nd ed. New York: John Wiley and Sons; 2016. p. 103–30.
Acknowledgments
The authors would like to thank Kyle Schaefer for the help in proofreading the manuscript.
Funding
This work was supported by a grant from the National Institutes of Health (1R15GM107197-01A1) and a scholarship from Saudi Arabian Cultural Mission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alqahtani, Y., Wang, S., Najmi, A. et al. Thiol-specific fluorogenic agent for live cell non-protein thiol imaging in lysosomes. Anal Bioanal Chem 411, 6463–6473 (2019). https://doi.org/10.1007/s00216-019-02026-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02026-3