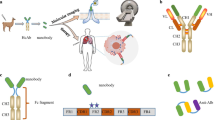
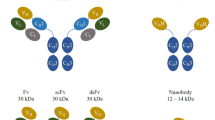
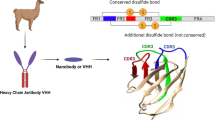
Abstract
Nanobodies (Nbs) have arisen as an alternative to conventional antibodies (Abs) and show great potential when used as tools in different biotechnology fields such as diagnostics and therapy. Different approaches have been described for the production of Nbs and these methods face new challenges focused on improving yield, affinity, and reducing production costs. This review summarizes these challenges, and also the latest advances in the detection of different kinds of molecules, such as proteins and small molecules, and describes their potential use for noninvasive in vivo imaging and for in vitro assays. Moreover, the unique properties of Nbs are outlined like internalization, size, thermal and chemical stability, affinity, blood clearance, and labeling procedures. Concerning therapeutic applications, we highlight some already reported examples about Nbs being used for the treatment of several diseases such as cancer, neurodegenerative or infectious diseases among others. Finally, future trends, opportunities, and disadvantages are also discussed.



Similar content being viewed by others
References
Ruigrok Vincent JB, Levisson M, Eppink Michel HM, Smidt H, van der Oost J. Alternative affinity tools: more attractive than antibodies? Biochem J. 2011;436(1):1–13. https://doi.org/10.1042/bj20101860.
Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem. 2013;82(1):775–97. https://doi.org/10.1146/annurev-biochem-063011-092449.
Ingram JR, Schmidt FI, Ploegh HL. Exploiting nanobodies’ singular traits. In: Littman DR, Yokoyama WM, editors. Annual review of immunology, vol 36. Annu rev Immunol. 2018; 695–715. https://doi.org/10.1146/annurev-immunol-042617-053327.
Hamers-Casterman C, Atarhouch T, Muyldermans S, Robinson G, Hamers C, Songa EB, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993;363(6428):446–8. https://doi.org/10.1038/363446a0.
Muyldermans S, Baral TN, Retamozzo VC, De Baetselier P, De Genst E, Kinne J, Leonhardt H, Magez S, Nguyen VK, Revets H, Rothbauer U, Stijlemans B, Tillib S, Wernery U, Wyns L, Hassanzadeh-Ghassabeh G, Saerens D. Camelid immunoglobulins and nanobody technology. Vet Immunol Immunopathol. 2009;128:178–183. https://doi.org/10.1016/j.vetimm.2008.10.299.
Steeland S, Vandenbroucke RE, Libert C. Nanobodies as therapeutics: big opportunities for small antibodies. Drug Discov Today. 2016;21(7):1076–113. https://doi.org/10.1016/j.drudis.2016.04.003.
Liu W, Song H, Chen Q, Yu J, Xian M, Nian R, et al. Recent advances in the selection and identification of antigen-specific nanobodies. Mol Immunol. 2018;96:37–47. https://doi.org/10.1016/j.molimm.2018.02.012.
Kubala MH, Kovtun O, Alexandrov K, Collins BM. Structural and thermodynamic analysis of the GFP:GFP-nanobody complex. Protein Sci. 2010;19(12):2389–401. https://doi.org/10.1002/pro.519.
Kunz P, Zinner K, Mücke N, Bartoschik T, Muyldermans S, Hoheisel JD. The structural basis of nanobody unfolding reversibility and thermoresistance. Sci Rep. 2018;8(1):7934. https://doi.org/10.1038/s41598-018-26338-z.
Siontorou CG. Nanobodies as novel agents for disease diagnosis and therapy. Int J Nanomed. 2013;8:4215–27. https://doi.org/10.2147/ijn.s39428.
Crivianu-Gaita V, Thompson M. Aptamers, antibody scFv, and antibody Fab’ fragments: an overview and comparison of three of the most versatile biosensor biorecognition elements. Biosens Bioelectron. 2016;85:32–45. https://doi.org/10.1016/j.bios.2016.04.091.
Wang Y, Fan Z, Shao L, Kong X, Hou X, Tian D, et al. Nanobody-derived nanobiotechnology tool kits for diverse biomedical and biotechnology applications. Int J Nanomed. 2016;11:3287–302. https://doi.org/10.2147/ijn.s107194.
Iezzi ME, Policastro L, Werbajh S, Podhajcer O, Canziani GA. Single-domain antibodies and the promise of modular targeting in cancer imaging and treatment. Front Immunol. 2018;9:273. https://doi.org/10.3389/fimmu.2018.00273.
Pardon E, Laeremans T, Triest S, Rasmussen SGF, Wohlkönig A, Ruf A, et al. A general protocol for the generation of nanobodies for structural biology. Nat Protocols. 2014;9(3):674–93. https://doi.org/10.1038/nprot.2014.039.
Liu Y, Huang H. Expression of single-domain antibody in different systems. Appl Microbiol Biotechnol. 2018;102(2):539–51. https://doi.org/10.1007/s00253-017-8644-3.
Sockolosky JT, Dougan M, Ingram JR, Ho CCM, Kauke MJ, Almo SC, et al. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci. 2016;113(19):E2646–54. https://doi.org/10.1073/pnas.1604268113.
Vuchelen A, O’Day E, De Genst E, Pardon E, Wyns L, Dumoulin M, et al. (1)H, (13)C and (15)N assignments of a camelid nanobody directed against human alpha-synuclein. Biomol NMR Assign. 2009;3(2):231–3. https://doi.org/10.1007/s12104-009-9182-4.
Kumar H, Finer-Moore JS, Jiang X, Smirnova I, Kasho V, Pardon E, et al. Crystal structure of a ligand-bound LacY-Nanobody complex. Proc Natl Acad Sci U S A. 2018;115(35):8769–74. https://doi.org/10.1073/pnas.1801774115.
Robert B, Dorvillius M, Buchegger F, Garambois V, Mani JC, Pugnieres M, et al. Tumor targeting with newly designed biparatopic antibodies directed against two different epitopes of the carcinoembryonic antigen (CEA). Int J Cancer. 1999;81(2):285–91.
Els Conrath K, Lauwereys M, Wyns L, Muyldermans S. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J Biol Chem. 2001;276(10):7346–50. https://doi.org/10.1074/jbc.M007734200.
Sedykh SE, Prinz VV, Buneva VN, Nevinsky GA. Bispecific antibodies: design, therapy, perspectives. Drug Design Dev Ther. 2018;12:195–208. https://doi.org/10.2147/DDDT.S151282.
Davies J, Riechmann L. Single antibody domains as small recognition units: design and in vitro antigen selection of camelized, human VH domains with improved protein stability. Protein Eng. 1996;9(6):531–7.
Arbabi Ghahroudi M, Desmyter A, Wyns L, Hamers R, Muyldermans S. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997;414(3):521–6.
Perez JM, Renisio JG, Prompers JJ, van Platerink CJ, Cambillau C, Darbon H, et al. Thermal unfolding of a llama antibody fragment: a two-state reversible process. Biochemistry. 2001;40(1):74–83.
Dumoulin M, Conrath K, Van Meirhaeghe A, Meersman F, Heremans K, Frenken LG, et al. Single-domain antibody fragments with high conformational stability. Protein Sci. 2002;11(3):500–15. https://doi.org/10.1110/ps.34602.
Wolfgang WJ, Miller TW, Webster JM, Huston JS, Thompson LM, Marsh JL, et al. Suppression of Huntington’s disease pathology in Drosophila by human single-chain Fv antibodies. Proc Natl Acad Sci U S A. 2005;102(32):11563–8. https://doi.org/10.1073/pnas.0505321102.
Wang J, Mukhtar H, Ma L, Pang Q, Wang X. VHH antibodies: reagents for mycotoxin detection in food products. Sensors. 2018;18(2):485.
McMurphy T, Xiao R, Magee D, Slater A, Zabeau L, Tavernier J, et al. The anti-tumor activity of a neutralizing nanobody targeting leptin receptor in a mouse model of melanoma. PLoS One. 2014;9(2):e89895. https://doi.org/10.1371/journal.pone.0089895.
Blick SK, Curran MP. Certolizumab pegol: in Crohn’s disease. BioDrugs. 2007;21(3):195–201; discussion 202-193. https://doi.org/10.2165/00063030-200721030-00006.
Padlan EA. X-ray crystallography of antibodies. Adv Protein Chem. 1996;49:57–133.
Conrath KE, Lauwereys M, Galleni M, Matagne A, Frere JM, Kinne J, Wyns L, Muyldermans S. Beta-lactamase inhibitors derived from single-domain antibody fragments elicited in the Camelidae. Antimicrob Agents Chemother. 2001;45(10):2807–2812. https://doi.org/10.1128/aac.45.10.2807-2812.2001.
Lauwereys M, Arbabi Ghahroudi M, Desmyter A, Kinne J, Holzer W, De Genst E, et al. Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J. 1998;17(13):3512–20. https://doi.org/10.1093/emboj/17.13.3512.
Vincke C, Loris R, Saerens D, Martinez-Rodriguez S, Muyldermans S, Conrath K. General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. J Biol Chem. 2009;284(5):3273–84. https://doi.org/10.1074/jbc.M806889200.
Saerens D, Huang L, Bonroy K, Muyldermans S. Antibody fragments as probe in biosensor development. Sensors (Basel). 2008;8(8):4669–86. https://doi.org/10.3390/s8084669.
Pinto Torres JE, Goossens J, Ding J, Li Z, Lu S, Vertommen D, et al. Development of a nanobody-based lateral flow assay to detect active Trypanosoma congolense infections. Sci Rep. 2018;8(1):9019. https://doi.org/10.1038/s41598-018-26732-7.
Qiu Y, Li P, Dong S, Zhang X, Yang Q, Wang Y, et al. Phage-mediated competitive chemiluminescent immunoassay for detecting Cry1Ab toxin by using an anti-idiotypic camel nanobody. J Agric Food Chem. 2018;66(4):950–6. https://doi.org/10.1021/acs.jafc.7b04923.
Tu Z, Chen Q, Li Y, Xiong Y, Xu Y, Hu N, et al. Identification and characterization of species-specific nanobodies for the detection of Listeria monocytogenes in milk. Anal Biochem. 2016;493:1–7. https://doi.org/10.1016/j.ab.2015.09.023.
Zafra O, Fraile S, Gutiérrez C, Haro A, Páez-Espino AD, Jiménez JI, et al. Monitoring biodegradative enzymes with nanobodies raised in Camelus dromedarius with mixtures of catabolic proteins. Environ Microbiol. 2011;13(4):960–74. https://doi.org/10.1111/j.1462-2920.2010.02401.x.
Campuzano S, Salema V, Moreno-Guzmán M, Gamella M, Yáñez-Sedeño P, Fernández LA, et al. Disposable amperometric magnetoimmunosensors using nanobodies as biorecognition element. Determination of fibrinogen in plasma. Biosens Bioelectron. 2014;52:255–60. https://doi.org/10.1016/j.bios.2013.08.055.
Marco M-P, Gee S, Hammock BD. Immunochemical techniques for environmental analysis II. Antibody production and immunoassay development. TrAC Trends Anal Chem. 1995;14(8):415–25. https://doi.org/10.1016/0165-9936(95)90920-I.
Fodey T, Leonard P, O’Mahony J, O’Kennedy R, Danaher M. Developments in the production of biological and synthetic binders for immunoassay and sensor-based detection of small molecules. TrAC Trends Anal Chem. 2011;30(2):254–69. https://doi.org/10.1016/j.trac.2010.10.011.
Bever CS, Dong J-X, Vasylieva N, Barnych B, Cui Y, Xu Z-L, et al. VHH antibodies: emerging reagents for the analysis of environmental chemicals. Anal Bioanal Chem. 2016;408(22):5985–6002. https://doi.org/10.1007/s00216-016-9585-x.
Alvarez-Rueda N, Behar G, Ferré V, Pugnière M, Roquet F, Gastinel L, et al. Generation of llama single-domain antibodies against methotrexate, a prototypical hapten. Mol Immunol. 2007;44(7):1680–90. https://doi.org/10.1016/j.molimm.2006.08.007.
Makvandi-Nejad S, Fjällman T, Arbabi-Ghahroudi M, MacKenzie CR, Hall JC. Selection and expression of recombinant single domain antibodies from a hyper-immunized library against the hapten azoxystrobin. J Immunol Methods. 2011;373(1):8–18. https://doi.org/10.1016/j.jim.2011.07.006.
Wang J, Bever CRS, Majkova Z, Dechant JE, Yang J, Gee SJ, et al. Heterologous antigen selection of camelid heavy chain single domain antibodies against tetrabromobisphenol A. Anal Chem. 2014;86(16):8296–302. https://doi.org/10.1021/ac5017437.
Kim H-J, McCoy MR, Majkova Z, Dechant JE, Gee SJ, Tabares-da Rosa S, et al. Isolation of alpaca anti-hapten heavy chain single domain antibodies for development of sensitive immunoassay. Anal Chem. 2012;84(2):1165–71. https://doi.org/10.1021/ac2030255.
Pan D, Li G, Hu H, Xue H, Zhang M, Zhu M, et al. Direct immunoassay for facile and sensitive detection of small molecule aflatoxin B1 based on nanobody. Chemistry. 2018;24(39):9869–76. https://doi.org/10.1002/chem.201801202.
Liu X, Tang Z, Duan Z, He Z, Shu M, Wang X, et al. Nanobody-based enzyme immunoassay for ochratoxin a in cereal with high resistance to matrix interference. Talanta. 2017;164:154–8. https://doi.org/10.1016/j.talanta.2016.11.039.
Wang J, Majkova Z, Bever CRS, Yang J, Gee SJ, Li J, et al. One-step immunoassay for tetrabromobisphenol A using a camelid single domain antibody–alkaline phosphatase fusion protein. Anal Chem. 2015;87(9):4741–8. https://doi.org/10.1021/ac504735p.
Tang X, Li P, Zhang Q, Zhang Z, Zhang W, Jiang J. Time-resolved fluorescence immunochromatographic assay developed using two idiotypic nanobodies for rapid, quantitative, and simultaneous detection of aflatoxin and zearalenone in maize and its products. Anal Chem. 2017;89(21):11520–8. https://doi.org/10.1021/acs.analchem.7b02794.
Traenkle B, Rothbauer U. Under the microscope: single-domain antibodies for live-cell imaging and super-resolution microscopy. Front Immunol. 2017;8:1030. https://doi.org/10.3389/fimmu.2017.01030.
Roeder R, Helma J, Prei T, Raedler JO, Leonhardt H, Wagner E. Intracellular delivery of nanobodies for imaging of target proteins in live cells. Pharm Res. 2017;34(1):161–74. https://doi.org/10.1007/s11095-016-2052-8.
Schumacher D, Helma J, Schneider AFL, Leonhardt H, Hackenberger CPR. Nanobodies: chemical functionalization strategies and intracellular applications. Angew Chem Int Ed. 2018;57(9):2314–33. https://doi.org/10.1002/anie.201708459.
Gainkam LOT, Huang L, Caveliers V, Keyaerts M, Hernot S, Vaneycken I, et al. Comparison of the biodistribution and tumor targeting of two Tc-99m-labeled anti-EGFR nanobodies in mice, using pinhole SPECT/micro-CT. J Nucl Med. 2008;49(5):788–95. https://doi.org/10.2967/jnumed.107.048538.
Debie P, Van Quathem J, Hansen I, Bala G, Massa S, Devoogdt N, et al. Effect of dye and conjugation chemistry on the biodistribution profile of near-infrared-labeled nanobodies as tracers for image-guided surgery. Mol Pharm. 2017;14(4):1145–53. https://doi.org/10.1021/acs.molpharmaceut.6b01053.
Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–7.
Fraser G, Smith CA, Imrie K, Meyer R, Hematology Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care. Alemtuzumab in chronic lymphocytic leukemia. Curr Oncol. 2007;14(3):96–109.
Casadevall A. The case for pathogen-specific therapy. Expert Opin Pharmacother. 2009;10(11):1699–703. https://doi.org/10.1517/14656560903066837.
Pankhurst T, Adu D. Antibodies in the prevention of renal allograft rejection. Expert Opin Biol Ther. 2004;4(2):243–52. https://doi.org/10.1517/14712598.4.2.243.
Ibanez LI, De Filette M, Hultberg A, Verrips T, Temperton N, Weiss RA, et al. Nanobodies with in vitro neutralizing activity protect mice against H5N1 influenza virus infection. J Infect Dis. 2011;203(8):1063–72. https://doi.org/10.1093/infdis/jiq168.
Unger M, Eichhoff AM, Schumacher L, Strysio M, Menzel S, Schwan C, et al. Selection of nanobodies that block the enzymatic and cytotoxic activities of the binary Clostridium difficile toxin CDT. Sci Rep. 2015;5:7850. https://doi.org/10.1038/srep07850.
Lafaye P, Achour I, England P, Duyckaerts C, Rougeon F. Single-domain antibodies recognize selectively small oligomeric forms of amyloid beta, prevent Abeta-induced neurotoxicity and inhibit fibril formation. Mol Immunol. 2009;46(4):695–704. https://doi.org/10.1016/j.molimm.2008.09.008.
Acknowledgements
This work has been funded by the ImmunoQS project funded by MINECO, Programa Estatal de Investigación Desarrollo e Innovación Orientada a los Retos de la Sociedad (SAF2015-67476-R). The Nb4D group is a consolidated research group (Grup de Recerca) of the Generalitat de Catalunya and has support from the Departament d’Universitats, Recerca i Societat de la Informació de la Generalitat de Catalunya (expedient: 2017 SGR 1441). CIBER-BBN is an initiative funded by the Spanish National Plan for Scientific and Technical Research and Innovation 2013-2016, Iniciativa Ingenio 2010, Consolider Program, CIBER Actions are financed by the Instituto de Salud Carlos III with assistance from the European Regional Development Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salvador, JP., Vilaplana, L. & Marco, MP. Nanobody: outstanding features for diagnostic and therapeutic applications. Anal Bioanal Chem 411, 1703–1713 (2019). https://doi.org/10.1007/s00216-019-01633-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01633-4