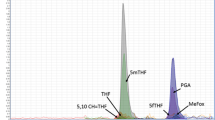
Abstract
Since the introduction of liquid chromatography tandem mass spectrometry in clinical laboratories, folate analysis has shifted from microbiological or protein-binding assays to chromatographic methods. Now, it is possible to sensitively and selectively determine several folate species in clinical samples where only a total folate content could be quantified using a microbiological or a binding assay. Although several chromatographic methods have been developed, validated, and published, interlaboratory variability limits the comparability of the results. In this review, we provide an overview of the latest strategies for sampling, sample treatment, and analysis and how these may influence the final analytical result. Among the variables covered are the effect of pH, temperature, and storage and the use of antioxidants and anticoagulants on analyte stability. In addition, we highlight the importance of correct assay calibration and the use of (labeled) certified reference materials in order to obtain correct and comparable results among different laboratories.

ᅟ



Similar content being viewed by others
References
De Brouwer V, Zhang GF, Storozhenko S, Straeten DV, Lambert WE. pH stability of individual folates during critical sample preparation steps in prevision of the analysis of plant folates. Phytochem Anal. 2007;18(6):496–508. https://doi.org/10.1002/pca.1006.
Osborne CB, Lowe KE, Shane B. Regulation of folate and one-carbon metabolism in mammalian cells. I. Folate metabolism in Chinese hamster ovary cells expressing Escherichia coli or human folylpoly-gamma-glutamate synthetase activity. J Biol Chem. 1993;268(29):21657–64.
Scott JM. Folate and vitamin B-12. Proc Nutr Soc. 1999;58(2):441–8. https://doi.org/10.1017/S0029665199000580.
Stover PJ. In: Bailey LB, editor. Folate biochemical pathways and their regulation, in folate in health and disease, vol. 2. Boca-Raton: CRC Press; 2009. p. 49–74.
Blom HJ, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis. 2011;34(1):75–81. https://doi.org/10.1007/s10545-010-9177-4.
Ueland PM, Refsum H, Beresford SA, Vollset SE. The controversy over homocysteine and cardiovascular risk. Am J Clin Nutr. 2000;72(2):324–32. https://doi.org/10.1093/ajcn/72.2.324.
Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006;5(11):949–60. https://doi.org/10.1016/S1474-4422(06)70598-1.
Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338(8760):131–137.
Blount BC, Mack MM, Wehr CM, MacGregor JT, Hiatt RA, Wang G, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94(7):3290–5. https://doi.org/10.1073/pnas.94.7.3290.
Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008;4:CD004514. https://doi.org/10.1002/14651858.CD004514.pub2.
Moran RG, Colman PD. Measurement of folylpolyglutamate synthetase in mammalian tissues. Anal Biochem. 1984;140(2):326–42. https://doi.org/10.1016/0003-2697(84)90174-X.
van Haandel L, Becker ML, Williams TD, Stobaugh JF, Leeder JS. Comprehensive quantitative measurement of folate polyglutamates in human erythrocytes by ion pairing ultra-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2012;26(14):1617–30. https://doi.org/10.1002/rcm.6268.
Kiekens F, Van Daele J, Blancquaert D, Van Der Straeten D, Lambert WE, Stove CP. A validated ultra-high-performance liquid chromatography-tandem mass spectrometry method for the selective analysis of free and total folate in plasma and red blood cells. J Chromatogr A. 2015;1398:20–8. https://doi.org/10.1016/j.chroma.2015.04.025.
Wang C, Riedl KM, Schwartz SJ. A liquid chromatography-tandem mass spectrometric method for quantitative determination of native 5-methyltetrahydrofolate and its polyglutamyl derivatives in raw vegetables. J Chromatogr B Anal Technol Biomed Life Sci. 2010;878(29):2949–58. https://doi.org/10.1016/j.jchromb.2010.08.043.
Allen LH. Causes of vitamin B-12 and folate deficiency. Food Nutr Bull. 2008;29(2):S20–34. https://doi.org/10.1177/15648265080292s105.
Lambie DG, Johnson RH. Drugs and folate metabolism. Drugs. 1985;30(2):145–55. https://doi.org/10.2165/00003495-198530020-00003.
Medici V, Halsted CH. Folate, alcohol, and liver disease. Mol Nutr Food Res. 2013;57(4):596–606. https://doi.org/10.1002/mnfr.201200077.
Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10(1):111–3. https://doi.org/10.1038/ng0595-111.
Franco RF, Araujo AG, Guerreiro JF, Elion J, Zago MA. Analysis of the 677 C-->T mutation of the methylenetetrahydrofolate reductase gene in different ethnic groups. Thromb Haemost. 1998;79(1):119–21. https://doi.org/10.1055/s-0037-1614230.
DeVos L, Chanson A, Liu Z, Ciappio ED, Parnell LD, Mason JB, et al. Associations between single nucleotide polymorphisms in folate uptake and metabolizing genes with blood folate, homocysteine, and DNA uracil concentrations. Am J Clin Nutr. 2008;88(4):1149–58. https://doi.org/10.1093/ajcn/88.4.1149.
WHO. Serum and red blood cell folate concentrations for assessing folate status in populations. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization; 2015.
WHO. Nutritional anaemias. In: Report of WHO grup of experts. Geneva: World Health Organization; 1972.
WHO. Control of nutritional anaemia with special reference to iron deficiency. Report of an IAEA/USAIS/WHO joint meeting, World Health Organization; 1975.
Selhub J, Jacques PF, Dallal G, Choumenkovitch S, Rogers G. The use of blood concentrations of vitamins and their respective functional indicators to define folate and vitamin B12 status. Food Nutr Bull. 2008;29(2 Suppl):S67–73. https://doi.org/10.1177/15648265080292S110.
Pfeiffer CM, Hughes JP, Lacher DA, Bailey RL, Berry RJ, Zhang M, et al. Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988-2010. J Nutr. 2012;142(5):886–93. https://doi.org/10.3945/jn.111.156919.
WHO. Guideline: optimal serum and red blood cell folate concentration in women of reproductive age for prevention of neural tube defects, World Health Organization; 2015.
Pfeiffer CM, Sternberg MR, Hamner HC, Crider KS, Lacher DA, Rogers LM, et al. Applying inappropriate cutoffs leads to misinterpretation of folate status in the US population. Am J Clin Nutr. 2016;104(6):1607–15. https://doi.org/10.3945/ajcn.116.138529.
Konings EJ, Troost FJ, Castenmiller JJ, Roomans HH, Van Den Brandt PA, Saris WH. Intestinal absorption of different types of folate in healthy subjects with an ileostomy. Br J Nutr. 2002;88(3):235–42. https://doi.org/10.1079/BJN2002613.
Bailey LB, Gregory JF 3rd. Folate metabolism and requirements. J Nutr. 1999;129(4):779–82. https://doi.org/10.1093/jn/129.4.779.
Pfeiffer CM, Sternberg MR, Fazili Z, Lacher DA, Zhang M, Johnson CL, et al. Folate status and concentrations of serum folate forms in the US population: National Health and Nutrition Examination Survey 2011-2. Br J Nutr. 2015;113(12):1965–77. https://doi.org/10.1017/S0007114515001142.
Strandler HS, Patring J, Jagerstad M, Jastrebova J. Challenges in the determination of unsubstituted food folates: impact of stabilities and conversions on analytical results. J Agric Food Chem. 2015;63(9):2367–77. https://doi.org/10.1021/jf504987n.
Akhtar MJ, Khan MA, Ahmad I. Photodegradation of folic acid in aqueous solution. J Pharm Biomed Anal. 1999;19(3–4):269–75. https://doi.org/10.1016/S0731-7085(98)00038-7.
Saubade F, Hemery YM, Guyot JP, Humblot C. Lactic acid fermentation as a tool for increasing the folate content of foods. Crit Rev Food Sci Nutr. 2016. https://doi.org/10.1080/10408398.2016.1192986.
Garbis SD, Melse-Boonstra A, West CE, van Breemen RB. Determination of folates in human plasma using hydrophilic interaction chromatography-tandem mass spectrometry. Anal Chem. 2001;73(22):5358–64. https://doi.org/10.1021/ac010741y.
Hart DJ, Finglas PM, Wolfe CA, Mellon F, Wright AJ, Southon S. Determination of 5-methyltetrahydrofolate (13C-labeled and unlabeled) in human plasma and urine by combined liquid chromatography mass spectrometry. Anal Biochem. 2002;305(2):206–13. https://doi.org/10.1006/abio.2002.5662.
Rychlik M, Netzel M, Pfannebecker I, Frank T, Bitsch I. Application of stable isotope dilution assays based on liquid chromatography-tandem mass spectrometry for the assessment of folate bioavailability. J Chromatogr B Anal Technol Biomed Life Sci. 2003;792(2):167–76. https://doi.org/10.1016/S1570-0232(03)00254-X.
Kok RM, Smith DE, Dainty JR, Van Den Akker JT, Finglas PM, Smulders YM, et al. 5-Methyltetrahydrofolic acid and folic acid measured in plasma with liquid chromatography tandem mass spectrometry: applications to folate absorption and metabolism. Anal Biochem. 2004;326(2):129–38. https://doi.org/10.1016/j.ab.2003.12.003.
Pfeiffer CM, Fazili Z, McCoy L, Zhang M, Gunter EW. Determination of folate vitamers in human serum by stable-isotope-dilution tandem mass spectrometry and comparison with radioassay and microbiologic assay. Clin Chem. 2004;50(2):423–32. https://doi.org/10.1373/clinchem.2003.026955.
Nelson BC, Pfeiffer CM, Margolis SA, Nelson CP. Solid-phase extraction-electrospray ionization mass spectrometry for the quantification of folate in human plasma or serum. Anal Biochem. 2004;325(1):41–51. https://doi.org/10.1016/j.ab.2003.10.009.
Huang Y, Khartulyari S, Morales ME, Stanislawska-Sachadyn A, Von Feldt JM, Whitehead AS, et al. Quantification of key red blood cell folates from subjects with defined MTHFR 677C>T genotypes using stable isotope dilution liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom. 2008;22(16):2403–12. https://doi.org/10.1002/rcm.3624.
Liu K, Dai X, Zhong D, Deng P, Ma J, Chen X. Simultaneous determination of 6R-leucovorin, 6S-leucovorin and 5-methyltetrahydrofolate in human plasma using solid phase extraction and chiral liquid chromatography-tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2009;877(10):902–10. https://doi.org/10.1016/j.jchromb.2009.02.046.
Hannisdal R, Ueland PM, Svardal A. Liquid chromatography-tandem mass spectrometry analysis of folate and folate catabolites in human serum. Clin Chem. 2009;55(6):1147–54. https://doi.org/10.1373/clinchem.2008.114389.
Monch S, Netzel M, Netzel G, Rychlik M. Quantitation of folates and their catabolites in blood plasma, erythrocytes, and urine by stable isotope dilution assays. Anal Biochem. 2010;398(2):150–60. https://doi.org/10.1016/j.ab.2009.11.007.
Kirsch SH, Knapp JP, Herrmann W, Obeid R. Quantification of key folate forms in serum using stable-isotope dilution ultra performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2010;878(1):68–75. https://doi.org/10.1016/j.jchromb.2009.11.021.
Fazili Z, Whitehead RD Jr, Paladugula N, Pfeiffer CM. A high-throughput LC-MS/MS method suitable for population biomonitoring measures five serum folate vitamers and one oxidation product. Anal Bioanal Chem. 2013;405(13):4549–60. https://doi.org/10.1007/s00216-013-6854-9.
Wang X, Zhang T, Zhao X, Guan Z, Wang Z, Zhu Z, et al. Quantification of folate metabolites in serum using ultraperformance liquid chromatography tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2014;962:9–13. https://doi.org/10.1016/j.jchromb.2014.05.023.
Zheng X-H, Jiang L-Y, Zhao L-T, Zhang Q-Y, Ding L. Simultaneous quantitation of folic acid and 5-methyltetrahydrofolic acid in human plasma by HPLC–MS/MS and its application to a pharmacokinetic study. J Pharm Anal. 2015;5:269–75. https://doi.org/10.1016/j.jpha.2015.05.004.
Kopp M, Rychlik M. Quantitation of 5-methyltetrahydrofolic acid in dried blood spots and dried plasma spots by stable isotope dilution assays. PLoS One. 2015;10(11):e0143639. https://doi.org/10.1371/journal.pone.0143639.
Guiraud SP, Montoliu I, Da Silva L, Dayon L, Galindo AN, Corthesy J, et al. High-throughput and simultaneous quantitative analysis of homocysteine-methionine cycle metabolites and co-factors in blood plasma and cerebrospinal fluid by isotope dilution LC-MS/MS. Anal Bioanal Chem. 2017;409(1):295–305. https://doi.org/10.1007/s00216-016-0003-1.
Fazili Z, Pfeiffer CM. Measurement of folates in serum and conventionally prepared whole blood lysates: application of an automated 96-well plate isotope-dilution tandem mass spectrometry method. Clin Chem. 2004;50(12):2378–81. https://doi.org/10.1373/clinchem.2004.036541.
Smith DE, Kok RM, Teerlink T, Jakobs C, Smulders YM. Quantitative determination of erythrocyte folate vitamer distribution by liquid chromatography-tandem mass spectrometry. Clin Chem Lab Med. 2006;44(4):450–9. https://doi.org/10.1515/CCLM.2006.085.
Kirsch SH, Herrmann W, Geisel J, Obeid R. Assay of whole blood (6S)-5-CH3-H4folate using ultra performance liquid chromatography tandem mass spectrometry. Anal Bioanal Chem. 2012;404(3):895–902. https://doi.org/10.1007/s00216-012-6180-7.
Kopp M, Rychlik M. Assessing volumetric absorptive microsampling coupled with stable isotope dilution assay and liquid chromatography-tandem mass spectrometry as potential diagnostic tool for whole blood 5-methyltetrahydrofolic acid. Front Nutr. 2017;4:9. https://doi.org/10.3389/fnut.2017.00009.
Nandania J, Kokkonen M, Euro L, Velagapudi V. Simultaneous measurement of folate cycle intermediates in different biological matrices using liquid chromatography-tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2018;1092:168–78. https://doi.org/10.1016/j.jchromb.2018.06.008.
O’Broin SD, Gunter EW. Screening of folate status with use of dried blood spots on filter paper. Am J Clin Nutr. 1999;70(3):359–67. https://doi.org/10.1093/ajcn/70.3.359.
Zimmerman RK, Slater ME, Langer EK, Ross JA, Spector LG. Long-term stability of folate in dried blood spots stored in several conditions. J Pediatr. 2013;163(2):596–597 e591. https://doi.org/10.1016/j.jpeds.2013.03.043.
De Kesel PM, Lambert WE, Stove CP. Does volumetric absorptive microsampling eliminate the hematocrit bias for caffeine and paraxanthine in dried blood samples? A comparative study. Anal Chim Acta. 2015;881:65–73. https://doi.org/10.1016/j.aca.2015.04.056.
De Kesel PM, Sadones N, Capiau S, Lambert WE, Stove CP. Hemato-critical issues in quantitative analysis of dried blood spots: challenges and solutions. Bioanalysis. 2013;5(16):2023–41. https://doi.org/10.4155/bio.13.156.
Kok MGM, Fillet M. Volumetric absorptive microsampling: current advances and applications. J Pharm Biomed Anal. 2018;147:288–96. https://doi.org/10.1016/j.jpba.2017.07.029.
Hannisdal R, Ueland PM, Eussen SJ, Svardal A, Hustad S. Analytical recovery of folate degradation products formed in human serum and plasma at room temperature. J Nutr. 2009;139(7):1415–8. https://doi.org/10.3945/jn.109.105635.
Ocke MC, Schrijver J, Obermann-de Boer GL, Bloemberg BP, Haenen GR, Kromhout D. Stability of blood (pro)vitamins during four years of storage at −20 degrees C: consequences for epidemiologic research. J Clin Epidemiol. 1995;48(8):1077–85. https://doi.org/10.1016/0895-4356(94)00232-F.
O'Broin JD, Temperley IJ, Scott JM. Erythrocyte, plasma, and serum folate: specimen stability before microbiological assay. Clin Chem. 1980;26(3):522–4.
O’Broin S, Kelleher B. Optimization of erythrocyte folate extraction. Clin Chem. 2001;47(12):2181–2.
Patring JDM, Johansson MS, Yazynina E, Jastrebova JA. Evaluation of impact of different antioxidants on stability of dietary folates during food sample preparation and storage of extracts prior to analysis. Anal Chim Acta. 2005;553(1–2):36–42. https://doi.org/10.1016/j.aca.2005.07.070.
Horne DW, Briggs WT, Wagner C. High-pressure liquid chromatographic separation of the naturally occurring folic acid monoglutamate derivatives. Anal Biochem. 1981;116(2):393–7. https://doi.org/10.1016/0003-2697(81)90378-X.
Horne DW. High-performance liquid chromatographic measurement of 5,10-methylenetetrahydrofolate in liver. Anal Biochem. 2001;297(2):154–9. https://doi.org/10.1006/abio.2001.5334.
Schittmayer M, Birner-Gruenberger R, Zamboni N. Quantification of cellular folate species by LC-MS after stabilization by derivatization. Anal Chem. 2018. https://doi.org/10.1021/acs.analchem.8b00650.
Fazili Z, Pfeiffer CM, Zhang M, Jain R. Erythrocyte folate extraction and quantitative determination by liquid chromatography-tandem mass spectrometry: comparison of results with microbiologic assay. Clin Chem. 2005;51(12):2318–25. https://doi.org/10.1373/clinchem.2005.053801.
Stamm RA, Fazili Z, Pfeiffer C. Addition of exogenous γ-glutamyl hydrolase eliminates the need for lengthy incubation of whole blood lysate for quantitation of folate vitamers by high performance liquid chromatography-tandem mass spectrometry. Curr Dev Nutr. 2017. https://doi.org/10.3945/cdn.117.002055.
Natsuhori M, Okada M, Ida R, Sasaki K, Shimoda M, Kokue E. Binding characteristics of folate to high affinity folate binding protein purified from porcine serum. J Vet Med Sci. 1999;61(7):743–8. https://doi.org/10.1292/jvms.61.743.
van Eijsden M, van der Wal MF, Hornstra G, Bonsel GJ. Can whole-blood samples be stored over 24 hours without compromising stability of C-reactive protein, retinol, ferritin, folic acid, and fatty acids in epidemiologic research? Clin Chem. 2005;51(1):230–2. https://doi.org/10.1373/clinchem.2004.042234.
Zhang DJ, Elswick RK, Miller WG, Bailey JL. Effect of serum-clot contact time on clinical chemistry laboratory results. Clin Chem. 1998;44(6 Pt 1):1325–33.
Drammeh BS, Schleicher RL, Pfeiffer CM, Jain RB, Zhang M, Nguyen PH. Effects of delayed sample processing and freezing on serum concentrations of selected nutritional indicators. Clin Chem. 2008;54(11):1883–91. https://doi.org/10.1373/clinchem.2008.108761.
Fazili Z, Sternberg MR, Paladugula N, Whitehead RD Jr, Chen H, Pfeiffer CM. The loss of 5-methyltetrahydrofolate in human serum under suboptimal preanalytical conditions can only partially be recovered by an oxidation product. J Nutr. 2014;144(11):1873–9. https://doi.org/10.3945/jn.114.198358.
Hannisdal R, Gislefoss RE, Grimsrud TK, Hustad S, Morkrid L, Ueland PM. Analytical recovery of folate and its degradation products in human serum stored at −25 degrees C for up to 29 years. J Nutr. 2010;140(3):522–6. https://doi.org/10.3945/jn.109.116418.
Jansen EH, Beekhof PK, Cremers JW, Schenk E. Long-term (in)stability of folate and vitamin B12 in human serum. Clin Chem Lab Med. 2012;50(10):1761–3. https://doi.org/10.1515/cclm-2012-0108.
Paladugula N, Fazili Z, Sternberg MR, G G, Pfeiffer C. Serum folate forms are stable during repeated analysis in the presence of ascorbic acid and during frozen sample storage. J Appl Lab Med. 2018;3(3). https://doi.org/10.1373/jalm.2018.027102.
Pfeiffer CM, Fazili Z, Zhang M. Folate analytical methodology. In: Folate in health and disease, vol. 2. Boca-Raton: CRC Press; 2010.
Selhub J. Determination of tissue folate composition by affinity chromatography followed by high-pressure ion pair liquid chromatography. Anal Biochem. 1989;182(1):84–93. https://doi.org/10.1016/0003-2697(89)90722-7.
Gregory JF 3rd. Chemical and nutritional aspects of folate research: analytical procedures, methods of folate synthesis, stability, and bioavailability of dietary folates. Adv Food Nutr Res. 1989;33:1–101. https://doi.org/10.1016/S1043-4526(08)60126-6.
Thorpe SJ, Heath A, Blackmore S, Lee A, Hamilton M, O'Broin S, et al. International standard for serum vitamin B(12) and serum folate: international collaborative study to evaluate a batch of lyophilised serum for B(12) and folate content. Clin Chem Lab Med. 2007;45(3):380–6. https://doi.org/10.1515/CCLM.2007.072.
Fazili Z, Pfeiffer CM. Accounting for an isobaric interference allows correct determination of folate vitamers in serum by isotope dilution-liquid chromatography-tandem MS. J Nutr. 2013;143(1):108–13. https://doi.org/10.3945/jn.112.166769.
Owens JE, Holstege DM, Clifford AJ. High-throughput method for the quantitation of total folate in whole blood using LC-MS/MS. J Agric Food Chem. 2007;55(9):3292–7. https://doi.org/10.1021/jf063648p.
Patring JD, Lanina SA, Jastrebova JA. Applicability of alkyl-bonded ultra-pure silica stationary phases for gradient reversed-phase HPLC of folates with conventional and volatile buffers under highly aqueous conditions. J Sep Sci. 2006;29(6):889–904. https://doi.org/10.1002/jssc.200500481.
Vahteristo LT, Ollilainen V, Koivistoinen PE, Varo P. Improvements in the analysis of reduced folate monoglutamates and folic acid in food by high-performance liquid chromatography. J Agric Food Chem. 1996;44(2):477–82. https://doi.org/10.1021/jf9503467.
Ndaw S, Bergaentzle M, Aoude-Werner D, Lahely S, Hasselmann C. Determination of folates in foods by high-performance liquid chromatography with fluorescence detection after precolumn conversion to 5-methyltetrahydrofolates. J Chromatogr A. 2001;928(1):77–90. https://doi.org/10.1016/S0021-9673(01)01129-3.
Bagley PJ, Selhub J. Analysis of folate form distribution by affinity followed by reversed- phase chromatography with electrical detection. Clin Chem. 2000;46(3):404–11.
Patring JD, Jastrebova JA. Application of liquid chromatography-electrospray ionisation mass spectrometry for determination of dietary folates: effects of buffer nature and mobile phase composition on sensitivity and selectivity. J Chromatogr A. 2007;1143(1–2):72–82. https://doi.org/10.1016/j.chroma.2006.12.079.
Freisleben A, Schieberle P, Rychlik M. Specific and sensitive quantification of folate vitamers in foods by stable isotope dilution assays using high-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2003;376(2):149–56. https://doi.org/10.1007/s00216-003-1844-y.
De Brouwer V, Storozhenko S, Van de Steene JC, Wille SMR, Stove CP, Van Der Straeten D, et al. Optimisation and validation of a liquid chromatography-tandem mass spectrometry method for folates in rice. J Chromatogr A. 2008;1215(1–2):125–32. https://doi.org/10.1016/j.chroma.2008.11.004.
Pfeiffer CM, Zhang M, Jabbar S. Framework for laboratory harmonization of folate measurements in low- and middle-income countries and regions. Ann N Y Acad Sci. 2018;1414(1):96–108. https://doi.org/10.1111/nyas.13532.
Rogers LM, Cordero AM, Pfeiffer CM, Hausman DB, Tsang BL, De-Regil LM, et al. Global folate status in women of reproductive age: a systematic review with emphasis on methodological issues. Ann N Y Acad Sci. 2018;1431(1):35–57. https://doi.org/10.1111/nyas.13963.
Yetley EA, Pfeiffer CM, Phinney KW, Fazili Z, Lacher DA, Bailey RL, et al. Biomarkers of folate status in NHANES: a roundtable summary. Am J Clin Nutr. 2011;94(1):303s–12s. https://doi.org/10.3945/ajcn.111.013011.
Fazili Z, Pfeiffer CM, Zhang M, Jain RB, Koontz D. Influence of 5,10-methylenetetrahydrofolate reductase polymorphism on whole-blood folate concentrations measured by LC-MS/MS, microbiologic assay, and bio-rad radioassay. Clin Chem. 2008;54(1):197–201. https://doi.org/10.1373/clinchem.2007.096545.
Fazili Z, Pfeiffer CM, Zhang M. Comparison of serum folate species analyzed by LC-MS/MS with total folate measured by microbiologic assay and Bio-Rad radioassay. Clin Chem. 2007;53(4):781–4. https://doi.org/10.1373/clinchem.2006.078451.
Freisleben A, Schieberle P, Rychlik M. Syntheses of labeled vitamers of folic acid to be used as internal standards in stable isotope dilution assays. J Agric Food Chem. 2002;50(17):4760–8. https://doi.org/10.1021/jf025571k.
Stokvis E, Rosing H, Beijnen JH. Stable isotopically labeled internal standards in quantitative bioanalysis using liquid chromatography/mass spectrometry: necessity or not? Rapid Commun Mass Spectrom. 2005;19(3):401–7. https://doi.org/10.1002/rcm.1790.
Fazili Z, Sternberg MR, Paladugula N, Pfeiffer CM. Two international round-robin studies showed good comparability of 5-methyltetrahydrofolate but poor comparability of folic acid measured in serum by different high-performance liquid chromatography-tandem mass spectrometry methods. J Nutr. 2017;147(9):1815–25. https://doi.org/10.3945/jn.117.254144.
Blakley RL. The biochemistry of folic acid and related pteridines. Amsterdam: North-Holland Publishing Company; 1969. https://doi.org/10.1002/ange.19700821818.
Karayannis MI, Samios DN, Gousetis CP. A study of the molar absorptivity of ascorbic acid at different wavelengths and pH values. Anal Chim Acta. 1977;93(1):275–9. https://doi.org/10.1016/0003-2670(77)80032-9.
Satterfield MB, Sniegoski LT, Sharpless KE, Welch MJ, Hornikova A, Zhang NF, et al. Development of a new standard reference material: SRM 1955 (homocysteine and folate in human serum). Anal Bioanal Chem. 2006;385(3):612–22. https://doi.org/10.1007/s00216-006-0434-1.
Camara JE, Pritchett JS, Daniels YC, Phinney KW, Sander LC. Value assignment of candidate standard reference material® 3949 folate vitamers in frozen human serum by isotope-dilution LC-MS/MS, MSACL 2015 US; 2015.
Thorpe SJ, Sands D, Heath AB, Hamilton MS, Blackmore S, Barrowcliffe T. An international standard for whole blood folate: evaluation of a lyophilised haemolysate in an international collaborative study. Clin Chem Lab Med. 2004;42(5):533–9. https://doi.org/10.1515/CCLM.2004.090.
Funding
The folate research of the authors was supported by GOA 01G00409 and BOF18/GOA/042 (Bijzonder Onderzoeksfonds UGent), IWT.141529 (Agentschap voor Innovatie door Wetenschap en Technologie), and 1S61617N (Fonds voor Wetenschappelijk Onderzoek - Vlaanderen).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published in the topical collection Young Investigators in (Bio-)Analytical Chemistry with guest editors Erin Baker, Kerstin Leopold, Francesco Ricci, and Wei Wang.
Rights and permissions
About this article
Cite this article
Verstraete, J., Kiekens, F., Strobbe, S. et al. Clinical determination of folates: recent analytical strategies and challenges. Anal Bioanal Chem 411, 4383–4399 (2019). https://doi.org/10.1007/s00216-019-01574-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01574-y