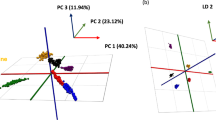
Abstract
Adult blow flies are one of the first necrophagous insects to colonize fresh carcasses. The eggs they lay hatch into larvae, which then feed on the decomposing body. Like all organisms, blow flies “are what they eat,” meaning that the isotopic composition of their body tissues reflects their diet. This manuscript combines ecology with a forensic application by using isotope ratio mass spectrometry (IRMS) to understand the relationship between the δ13C of amino acids in different carrion sources and the blow fly that feed on them. We also measure the amino acid-level fractionation that occurs at each major life stage of the blow flies. Adult blow flies from a commercial strain of Calliphora vicina (Robineau-Desvoidy) (Diptera: Calliphoridae) oviposited on raw pork muscle, beef muscle, or chicken liver. Larvae, pupae, and adult blow flies from each carrion were selected for amino acid compound-specific isotope analysis. Canonical discriminant analysis showed that flies were correctly classified to specific carrion types in 100% (original rules) and 96.8% (leave-one-out cross-validation [LOOCV]) of cases. Regarding life stages, we obtained 100% and 71% of correct classification in original rules and LOOCV, respectively. The isotope ratios of most of the essential amino acids did not significantly change between life stages (at 95% CI). However, some non-essential amino acids (Ala, Ser, and Glu) and some conditionally essential amino acids (Gly and Pro) were isotopically depleted in the adult stage. Except for the essential amino acids, the amino acids in larvae and pupae were enriched in 13C, and adult blow flies were depleted in 13C relative to the carrion on which they fed. These results make it possible to exclude potential sources of carrion as larval food. Amino acid-specific IRMS could help inform entomologists whether a fly has just arrived from another location to feed on a corpse or has emerged from a pupa whose feedstock was the corpse. Such insight could enhance the significance of blow flies for post-mortem interval determinations. The analytical ability to link organisms from one trophic level to another through the use of compound-specific isotope analysis of amino acids could have wide-reaching consequences in a variety of disciplines.

ᅟ






Similar content being viewed by others
References
Ostrom PH, Colunga-Garcia M, Gage SH. Establishing pathways of energy flow for insect predators using stable isotope ratios: field and laboratory evidence. Oecologia. 1997;109:108–13.
Gratton C, Forbes AE. Changes in delta δ 13C stable isotopes in multiple tissues of insect predators fed isotopically distinct prey. Oecologia. 2006;147(4):615–24.
Hood-Nowotny R, Knols BGJ. Stable isotope methods in biological and ecological studies of arthropods. Entomol Exp Appl. 2007;124(1):3–16.
Tibbets TM, Wheeless LA, del Rio CM. Isotopic enrichment without change in diet: an ontogenetic shift in δ 15N during insect metamorphosis. Funct Ecol 2008;22:109–113.
Wehi PM, Hicks BJ. Isotopic fractionation in a large herbivorous insect, the Auckland tree weta. J Insect Physiol. 2010;56(12):1877–82.
Hyodo F. Use of stable carbon and nitrogen isotopes in insect trophic ecology. Entomol Sci. 2015;18(3):295–312.
O'Brien DM, Fogel ML, Boggs CL. Renewable and nonrenewable resources: amino acid turnover and allocation to reproduction in Lepidoptera. Proc Natl Acad Sci U S A. 2002;99(7):4413–8.
O'Brien DM, Boggs CL, Fogel ML. Pollen feeding in the butterfly Heliconius charitonia: isotopic evidence for essential amino acid transfer from pollen to eggs. Proc Biol Sci. 2003;270(1533):2631–6.
Chikaraishi Y, Ogawa NO, Doi H, Ohkouchi N. 15N/14N ratios of amino acids as a tool for studying terrestrial food webs: a case study of terrestrial insects (bees, wasps, and hornets). Ecol Res. 2011;26(4):835–44.
Swift MJ, Heal OW, Anderson JM. Decomposition in terrestrial ecosystems. 1979. University of California Press, Berkeley and Los Angeles, CA.
Moore JC, Berlow EL, Coleman DC, Ruiter PC, Dong Q, Hastings A, et al. Detritus, trophic dynamics and biodiversity. Ecol Lett. 2004;7(7):584–600.
Ecology C. Evolution, and their applications. Boca Raton, FL: CRC Press; 2015.
Yang LH. Periodical cicadas as resource pulses in North American forests. Science. 2004;306:1565–7.
Forensic Entomology: the utility of arthropods in legal investigations. 2009. 2nd ed. edn. Taylor & Francis: Boca Raton.
Mohr RM, Tomberlin JK. Development and validation of a new technique for estimating a minimum postmortem interval using adult blow fly (Diptera: Calliphoridae) carcass attendance. Int J Legal Med. 2015;129(4):851–9.
Tarone AM, Sanford MR. Is PMI the hypothesis or the null hypothesis? J Med Entomol. 2017;54(5):1109–15.
Tomberlin JK, Mohr R, Benbow ME, Tarone AM, VanLaerhoven S. A roadmap for bridging basic and applied research in forensic entomology. Annu Rev Entomol. 2011;56:401–21.
Amendt J, Campobasso CP, Gaudry E, Reiter C, LeBlanc HN, Hall MJ. Best practice in forensic entomology—standards and guidelines. Int J Legal Med 2007;121(2):90–104.
Archer MS, Elgar MA, Briggs CA, Ranson DL. Fly pupae and puparia as potential contaminants of forensic entomology samples from sites of body discovery. Int J Legal Med. 2006;120(6):364–8.
Zehner R, Amendt J, Krettek R. STR typing of human DNA from fly larvae fed on decomposing bodies. J Forensic Sci. 2004;49(2):1–4.
Wells JD, Introna FJ, Di Vella G, Campobasso CP, Hayes J, Sperling FAH. Human and insect mitochondrial DNA analysis from maggots. J Forensic Sci 2001;46(3):685–687.
Hobson RP. Studies on the nutrition of blow-fly larvae: III. The liquefaction of muscle. J Exp Biol. 1932;9:359–65.
Njau DG, Muge EK, Kinyanjui PW, Omwandho COA, Mukwana S. STR analysis of human DNA from maggots fed on decomposing bodies: assessment of the time period for successful analysis. Egypt J Forensic Sci. 2016;6(3):261–9.
Marchetti D, Arena E, Boschi I, Vanin S. Human DNA extraction from empty puparia. Forensic Sci Int. 2013;229(1–3):e26–9.
Patt JM, Wainright SC, Hamilton GC, Whittinghill D, Bosley K, Dietrick J, et al. Assimilation of carbon and nitrogen from pollen and nectar by a predaceous larva and its effects on growth and development. Ecol Entomol. 2003;28:717–28.
Spence KO, Rosenheim JA. Isotopic enrichment in herbivorous insects: a comparative field-based study of variation. Oecologia. 2005;146:89–97.
Fraser I, Meier-Augenstein W, Kalin RM. The role of stable isotopes in human identification: a longitudinal study into the variability of isotopic signals in human hair and nails. Rapid Commun Mass Spectrom. 2006;20(7):1109–16.
Meier-Augenstein W, Fraser I. Forensic isotope analysis leads to identification of a mutilated murder victim. Sci Justice. 2008;48(3):153–9.
Mutzel Rauch E, Lehn C, Peschel O, Holzl S, Rossmann A. Assignment of unknown persons to their geographical origin by determination of stable isotopes in hair samples. Int J Legal Med. 2009;123(1):35–40.
Giffen JE, Rosati JY, Longo CM, Musah RA. Species identification of necrophagous insect eggs based on amino acid profile differences revealed by direct analysis in real time-high resolution mass spectrometry. Anal Chem. 2017;89(14):7719–26.
Webb SC, Hedges REM, Simpson SJ. Diet quality influences the δ 13C and δ 15N of locusts and their biochemical components. J Exp Biol. 1998;201:2903–11.
Jackson GP, An Y, Konstantynova KI, Rashaid AHB. Biometrics from the carbon isotope ratio analysis of amino acids in human hair. Sci Justice. 2015;55:43–50.
Carter JF, Fry B. Ensuring the reliability of stable isotope ratio data-beyond the principle of identical treatment. Anal Bioanal Chem. 2013;405(9):2799–814.
Coplen TB. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun Mass Spectrom. 2011;25:2538–60.
Dunn PJH, Carter JF. Good practice guide for isotope ratio mass spectrometry, 2nd Edition. 2018. FIRMS, 2nd edn.
McCullagh JS, Juchelka D, Hedges RE. Analysis of amino acid 13C abundance from human and faunal bone collagen using liquid chromatography/isotope ratio mass spectrometry. Rapid Commun Mass Spectrom. 2006;20(18):2761–8.
Godin JP, Fay LB, Hopfgartner G. Liquid chromatography combined with mass spectrometry for 13C isotopic analysis in life science research. Mass Spectrom Rev. 2007;26(6):751–74.
Smith CI, Fuller BT, Choy K, Richards MP. A three-phase liquid chromatographic method for delta C-13 analysis of amino acids from biological protein hydrolysates using liquid chromatography-isotope ratio mass spectrometry. Anal Biochem. 2009;390(2):165–72.
Raghavan M, McCullagh JSO, Lynnerup N, Hedges REM. Amino acid delta C-13 analysis of hair proteins and bone collagen using liquid chromatography/isotope ratio mass spectrometry: paleodietary implications from intra-individual comparisons. Rapid Commun Mass Spectrom. 2010;24(5):541–8.
McCullagh JS. Mixed-mode chromatography/isotope ratio mass spectrometry. Rapid Commun Mass Spectrom. 2010;24(5):483–94.
Godin JP, McCullagh JS. Review: current applications and challenges for liquid chromatography coupled to isotope ratio mass spectrometry (LC/IRMS). Rapid Commun Mass Spectrom. 2011;25(20):3019–28.
Jim S, Jones V, Copley MS, Ambrose SH, Evershed RP. Effects of hydrolysis on the δ 13C values of individual amino acids derived from polypeptides and proteins. Rapid Commun Mass Spectrom. 2003;17(20):2283–9.
Howland MR, Corr LT, Young SMM, Jones V, Jim S, Van Der Merwe NJ, Mitchell AD, Evershed RP. Expression of the dietary isotope signal in the compound-specific δ 13C values of pig bone lipids and amino acids. Int J Osteoarchaeol 2003;13(1–2):54–65.
DeNiro MJ, Epstein S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta. 1981;45:341–51.
Minagawa M, Wada E. Stepwise enrichment of 15N along food chains: further evidence and the relation between δ 15N and animal age. Geochim Cosmochim Acta. 1984;48:1135–40.
Post DM. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology. 2002;83(3):703–18.
Doi H, Kikuchi E, TaKagi S, Shikano S. Changes in carbon and nitrogen stable isotopes of chironomid larvae during growth, starvation and metamorphosis. Rapid communications in mass spectrometry : RCM 2007;21:997–1002.
McCutchan JH, Lewis WM, Kendall C, McGrath CC. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos. 2003;102:378–90.
Boecklen WJ, Yarnes CT, Cook BA, James AC. On the use of stable isotopes in trophic ecology. Annu Rev Ecol Evol Syst. 2011;42(1):411–40.
Hulsemann F, Lehn C, Schneider S, Jackson G, Hill S, Rossmann A, et al. Global spatial distributions of nitrogen and carbon stable isotope ratios of modern human hair. Rapid Commun Mass Spectrom. 2015;29(22):2111–21.
DeNiro MJ, Epstein S. Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta. 1978;42:495–506.
Peterson BJ, Fry B. Stable isotopes in ecosystem studies. Annu Rev Ecol Syst. 1987;18:293–320.
Tomberlin JK, Crippen TL, Tarone AM, Chaudhury MFB, Singh B, Cammack JA, et al. A review of bacterial interactions with blow flies (Diptera: Calliphoridae) of medical, veterinary, and forensic importance. Ann Entomol Soc Am. 2017;110(1):19–36.
McMahon KW, Polito MJ, Abel S, McCarthy MD, Thorrold SR. Carbon and nitrogen isotope fractionation of amino acids in an avian marine predator, the gentoo penguin (Pygoscelis papua). Ecol Evol. 2015;5(6):1278–90.
Langellotto GA, Rosenheim JA, Williams MR. Enhanced carbon enrichment in parasitoids (Hymenoptera): a stable isotope study. Ann Entomol Soc Am. 2005;98(2):205–13.
Jim S, Jones V, Ambrose SH, Evershed RP. Quantifying dietary macronutrient sources of carbon for bone collagen biosynthesis using natural abundance stable carbon isotope analysis. Br J Nutr. 2006;95(06):1055.
McMahon KW, Fogel ML, Elsdon TS, Thorrold SR. Carbon isotope fractionation of amino acids in fish muscle reflects biosynthesis and isotopic routing from dietary protein. J Anim Ecol. 2010;79(5):1132–41.
Chapman RF. The insects: structure and function. 2013. 5th edn. Cambridge University Press, New York.
Arrese EL, Soulages JL. Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol. 2010;55:207–25.
Law JH, Wells MA. Insects as biochemical models. J Biol Chem. 1989;264(28):16335–8.
Gilby AR. Lipids and their metabolism in insects. Annu Rev Entomol. 1965;10:141–60.
DeNiro MJ, Epstein S. Mechanism of carbon isotope fractionation associated with lipid synthesis. Science. 1977;197(4300):261–3.
Hayes JM. Factors controlling 13C contents of sedimentary organic compounds: principles and evidence. Mar Geol. 1993;113:111–25.
Tapiero H, Mathé G, Couvreur P, Tew KDII. Glutamine and glutamate. Biomed Pharmacother. 2002;56:446–57.
Hare PE, Fogel ML, Stafford TW, Mitchell AD, Hoering TC. The isotopic composition of carbon and nitrogen in individual amino acids isolated from modern and fossil proteins. J Archaeol Sci. 1991;18(3):277–92.
Reddy SRR, Campbell JW. Arginine metabolism in insects - role of arginase in proline formation during silkmoth development. Biochem J. 1969;115:495–503.
Acknowledgements
We would like to thank Dr. Rita Rio, from the Department of Biology at West Virginia University, for her valuable insights about amino acids and their metabolism in insects. We also appreciate the valuable feedback form the external reviewers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No human subjects were used in this research, and no live animals were used. Animal flesh (meat) was purchased from grocery stores.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 262 kb)
Rights and permissions
About this article
Cite this article
Matos, M.P.V., Konstantynova, K.I., Mohr, R.M. et al. Analysis of the 13C isotope ratios of amino acids in the larvae, pupae and adult stages of Calliphora vicina blow flies and their carrion food sources. Anal Bioanal Chem 410, 7943–7954 (2018). https://doi.org/10.1007/s00216-018-1416-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1416-9