Abstract
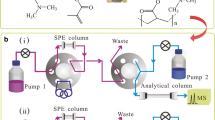
An automated on-line solid-phase extraction (SPE) following liquid chromatography tandem mass spectrometry was established for the fast determination of plant growth regulator residues in soybean sprout and mung bean sprout. The crude extracted specimens were directly purified on a poly (2-(dimethylamino) ethyl methacrylate-co-ethylene dimethacrylate) monolithic column which was well-defined as the on-line SPE adsorbent. Under the optimized conditions, the developed method gave the linear range of 0.3–50 ng/mL for gibberellin and 2,4-dichlorophenoxyacetic acid, 0.2–50 ng/mL for 4-chlorophenoxyacetic acid, and 0.5–50 ng/mL for 1-naphthaleneacetic acid (r ≥ 0.998). The detection limits (S/N = 3) ranged from 1.0 to 2.5 μg/kg and the recoveries for spiked soybean sprout samples were in the range of 75.0–93.3%. Besides, the total time for one analysis was 16 min. The reusability of the monolith was up to 600 extractions. The proposed process facilitated fully automated SPE and accurate determination in one step with rapidity, simplicity, and reliability.

ᅟ




Similar content being viewed by others
References
Calvo P, Nelson L, Kloepper JW. Agricultural uses of plant biostimulants. Plant Soil. 2014;383:3–41.
Rademacher W. Plant growth regulators: backgrounds and uses in plant production. J Plant Growth Regul. 2015;34:845–72.
Isik I, Celik I. Investigation of neurotoxic and immunotoxic effects of some plant growth regulators at subacute and subchronic applications on rats. Toxicol Ind Health. 2015;31:1095–105.
Li K, Wu JQ, Jiang LL, Shen LZ, Li JY, He ZH, et al. Developmental toxicity of 2,4-dichlorophenoxyacetic acid in zebrafish embryos. Chemosphere. 2017;171:40–8.
Wang KS, Lu CY, Chang SH. Evaluation of acute toxicity and teratogenic effects of plant growth regulators by Daphnia magna embryo assay. J Hazard Mater. 2011;190:520–8.
Australian pesticides and veterinary medicines authority. Agricultural and veterinary chemicals code instrument No 4 (MRL standard) 2012.2012. https://www.legislation.gov.au/Details/F2018C00105/. Accessed 23 Mar 2018.
Health and safety executive maximum residue level (MRL) database 2005. https://secure.pesticides.gov.uk/MRLs/main.asp/. Accessed 23 Mar 2018.
Ministry of Health, Labour and welfare. The Japanese positive list system for agricultural chemical residues in foods 2006 http://www.mhlw.go.jp/english/topics/foodsafety/positivelist060228/introduction.html. Accessed 23 Mar 2018.
National Health and Family Planning Commission of the People’s Republic of China. National food safety standard—maximum residue limits for pesticides in food. 2016. http://bz.cfsa.net.cn/staticPages/0D64FDAF-B210-43F4-B01D-8A97B276BCAF.html. Accessed 23 Mar 2018.
United States Environmental Protection Agency. Electronic code of federal regulations. 2018. https://www.ecfr.gov/cgi-bin/text-idx?SID=a518f1e8ebe11a4558132a9105399503&mc=true&tpl=/ecfrbrowse/Title40/40cfr180_main_02.tpl/. Accessed 23 Mar 2018.
Kestwal RM, Bagal-Kestwal D, Chiang BH. Analysis and enhancement of nutritional and antioxidant properties of Vigna aconitifolia sprouts. Plant Food Hum Nutr. 2012;67:136–41.
Cho SK, Abd El-Aty AM, Park KH, Park JH, Assayed ME, Jeong YM, et al. Simple multiresidue extraction method for the determination of fungicides and plant growth regulator in bean sprouts using low temperature partitioning and tandem mass spectrometry. Food Chem. 2013;136:1414–20.
Esparza X, Moyano E, Cosialls JR, Galceran MT. Determination of naphthalene-derived compounds in apples by ultra-high performance liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 2013;782:28–36.
Ma L, Zhang H, Xu W, He X, Yang L, Luo Y, et al. Simultaneous determination of 15 plant growth regulators in bean sprout and tomato with liquid chromatography-triple quadrupole tandem mass spectrometry. Food Anal Method. 2012;6:3158–65.
Wang X, Mao X, Yan A, Tan T, Yang Y, Wan Y. Simultaneous determination of nine plant growth regulators in navel oranges by liquid chromatography-triple quadrupole tandem mass spectrometry. Food Anal Method. 2016;9:3268–77.
Shi X, Jin F, Huang Y, Du X, Li C, Wang M, et al. Simultaneous determination of five plant growth regulators in fruits by modified quick, easy, cheap, effective, rugged, and safe (QuEChERS) extraction and liquid chromatography-tandem mass spectrometry. J Agr Food Chem. 2012;60:60–5.
Zhang F, Zhao P, Shan W, Gong Y, Jian Q, Pan C. Development of a method for the analysis of four plant growth regulators (PGRs) residues in soybean sprouts and mung bean sprouts by liquid chromatography-tandem mass spectrometry. B Environ Contam Tox. 2012;89:674–9.
Cao S, Zhou X, Li X, Tang B, Ding X, Xi C, et al. Determination of 17 plant growth regulator residues by ultra-high performance liquid chromatography-triple quadrupole linear ion trap mass spectrometry based on modified QuEChERS method. Food Anal Method. 2017;10:3158–65.
Gupta V, Kumar M, Brahmbhatt H, Reddy CR, Seth A, Jha B. Simultaneous determination of different endogenetic plant growth regulators in common green seaweeds using dispersive liquid-liquid microextraction method. Plant Physiol Biochem. 2011;49:1259–63.
Mao X, Tang L, Tan T, Wan Y. Determination of plant growth regulators in pears by microwave-assisted extraction and liquid chromatography with electrospray ionization mass spectrometry. J Sep Sci. 2014;37:1352–8.
Lu Q, Wu JH, Yu QW, Feng YQ. Using pollen grains as novel hydrophilic solid-phase extraction sorbents for the simultaneous determination of 16 plant growth regulators. J Chromatogr A. 2014;1367:39–47.
Moser C, Zoderer D, Luef G, Rauchenzauner M, Wildt L, Griesmacher A, et al. Simultaneous online SPE-LC-MS/MS quantification of six widely used synthetic progestins in human plasma. Anal Bioanal Chem. 2012;403:961–72.
Nunez O, Gallart-Ayala H, Martins CP, Lucci P. New trends in fast liquid chromatography for food and environmental analysis. J Chromatogr A. 2012;1228:298–323.
Ramirez CE, Wang C, Gardinali PR. Fully automated trace level determination of parent and alkylated PAHs in environmental waters by online SPE-LC-APPI-MS/MS. Anal Bioanal Chem. 2014;406:329–44.
Franco MS, Padovan RN, Fumes BH, Lancas FM. An overview of multidimensional liquid phase separations in food analysis. Electrophoresis. 2016;37:1768–83.
Pan J, Zhang C, Zhang Z, Li G. Review of online coupling of sample preparation techniques with liquid chromatography. Anal Chim Acta. 2014;815:1–15.
Chen L, Huang X. Sensitive monitoring of fluoroquinolones in milk and honey using multiple monolithic fiber solid-phase microextraction coupled to liquid chromatography tandem mass spectrometry. J Agr Food Chem. 2016;64:8684–93.
Fumes BH, Silva MR, Andrade FN, Nazario CED, Lanças FM. Recent advances and future trends in new materials for sample preparation. Trac Trend Anal Chem. 2015;71:9–25.
Wang TT, Chen YH, Ma JF, Hu MJ, Li Y, Fang JH, et al. A novel ionic liquid-modified organic-polymer monolith as the sorbent for in-tube solid-phase microextraction of acidic food additives. Anal Bioanal Chem. 2014;406:4955–63.
Liu Y, Wang MM, Ai LF, Zhang CK, Li X, Wang XS. Determination of Sudan dyes in chili pepper powder by online solid-phase extraction with a butyl methacrylate monolithic column coupled to liquid chromatography with tandem mass spectrometry. J Sep Sci. 2014;37:1648–55.
Svec F, Lv Y. Advances and recent trends in the field of monolithic columns for chromatography. Anal Chem. 2015;87:250–73.
Xu X, Duhoranimana E, Zhan X. Selective extraction of methenamine from chicken eggs using molecularly imprinted polymers and LC-MS/MS confirmation. Food Control. 2017;73:265–72.
Xu L, Shi ZG, Feng YQ. Porous monoliths: sorbents for miniaturized extraction in biological analysis. Anal Bioanal Chem. 2011;399:3345–57.
Jandera P. Advances in the development of organic polymer monolithic columns and their applications in food analysis - a review. J Chromatogr A. 2013;1313:37–53.
Masini JC, Svec F. Porous monoliths for on-line sample preparation: a review. Anal Chim Acta. 2017;964:24–44.
Candish E, Wirth HJ, Gooley AA, Shellie RA, Hilder EF. Characterization of large surface area polymer monoliths and their utility for rapid, selective solid phase extraction for improved sample clean up. J Chromatogr A. 2015;1410:9–18.
Ribeiro LF, Masini JC. Complexing porous polymer monoliths for online solid-phase extraction of metals in sequential injection analysis with electrochemical detection. Talanta. 2018;185:387–95.
Hu Y, Fan Y, Li G. Preparation and evaluation of a porous monolithic capillary column for microextraction of estrogens from urine and milk samples online coupled to high-performance liquid chromatography. J Chromatogr A. 2012;1228:205–12.
Lu X, Li G, Hu Y. In-tube solid-phase microextraction based on NH2-MIL-53(Al)-polymer monolithic column for online coupling with high-performance liquid chromatography for directly sensitive analysis of estrogens in human urine. Talanta. 2017;165:377–83.
Rogeberg M, Malerod H, Roberg-Larsen H, Aass C, Wilson SR. On-line solid phase extraction-liquid chromatography, with emphasis on modern bioanalysis and miniaturized systems. J Pharmaceut Biomed. 2014;87:120–9.
Yang Y, Mo F, Chen Y, Liu Y, Chen S, Zuo J. Preparation of 2-(dimethylamino) ethyl methacrylate copolymer micelles for shape memory materials. J Appl Polym Sci. 2015;132:42312.
Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–30.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21305028), the Natural Science Foundation of Hebei Province, China (No. H2017209232 and No.H2016209018), the Research Foundation of Education Bureau of Hebei Province, China (No. ZD2018014), and the Training Foundation of North China University of Science and Technology (No. JQ201717).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 61.5 kb)
Rights and permissions
About this article
Cite this article
Nian, Q., Ai, L., Li, D. et al. Rapid monitoring of plant growth regulators in bean sprouts via automated on-line polymeric monolith solid-phase extraction coupled with liquid chromatography tandem mass spectrometry. Anal Bioanal Chem 410, 7239–7247 (2018). https://doi.org/10.1007/s00216-018-1334-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1334-x