Abstract
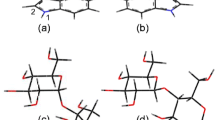
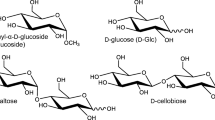
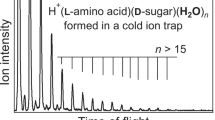
Chiral and molecular recognition between amino acid and sugar molecules and their implications for chemical evolution were investigated using a tandem mass spectrometer equipped with an electrospray ionization source and a cold ion trap. Ultraviolet photodissociation of mass-selected and temperature-controlled gas-phase noncovalent complexes of protonated tryptophan (Trp) and monosaccharide enantiomers, such as aldohexose, aldopentose, and deoxyhexose, was examined as a model for chemical evolution in interstellar molecular clouds. Upon photoexcitation of noncovalent heterochiral H+(l-Trp)(d-aldohexose) complexes, NH2CHCOOH loss from protonated Trp via Cα–Cβ bond cleavage occurred. Conversely, in homochiral H+(l-Trp)(l-aldohexose), the energy absorbed by Trp was released through the detachment of aldohexose, and dissociation of the amino acid was suppressed. In the photodissociation mass spectra of protonated Trp with aldopentose and deoxyhexose, which lacks the OH group of aldohexose, no dissociation of the molecules in the complexes or differences between enantiomers were observed. These results indicate that the OH groups in monosaccharides contribute to enantiomer-selective photodissociation in molecular clouds. The differences observed between enantiomers in the photodissociation mass spectra were applied to distinguishing and quantifying aldohexose enantiomers in solution using l-Trp as a chiral probe. The enantiomeric excesses of aldohexoses in solution could be determined from a single photodissociation mass spectrum by reference to the relative ion intensities for the NH2CHCOOH-elimination product and H+(l-Trp) formed via detachment of aldohexose. This analysis method could also distinguish and quantify two d-aldohexose mixtures, where l-Trp was employed as an isomer probe.

ᅟ







Similar content being viewed by others
References
Srinivas NR. Evaluation of experimental strategies for the development of chiral chromatographic methods based on diastereomer formation. Biomed Chromatogr. 2004;18:207–33.
McConnell O, Bach A, Balibar C, Byrne N, Cai Y, Carter G, et al. Enantiomeric separation and determination of absolute stereochemistry of asymmetric molecules in drug discovery—building chiral technology toolboxes. Chirality. 2007;19:658–82.
Flack HD, Bernardinelli G. The use of X-ray crystallography to determine absolute configuration. Chirality. 2008;20:681–90.
Nagata H, Machida Y, Nishi H, Kamigauchi M, Minoura K, Ishida T. Structural requirement for chiral recognition of amino acid by (18-crown-6)-tetracarboxylic acid: binding analysis in solution and solid states. Bull Chem Soc Jpn. 2009;82:219–29.
Ward TJ, Ward KD. Chiral separations: a review of current topics and trends. Anal Chem. 2012;84:626–35.
Myrgorodska I, Javelle T, Meinert C, Meierhenrich UJ. Enantioresolution and quantification of monosaccharides by comprehensive two-dimensional gas chromatography. J Chromatogr A. 2017;1487:248–53.
Hulst AG, Kientz CE. Differentitation between the isomeric amino acids leucine and isoleucine using low-energy collision-induced dissociation tandem mass spectroscopy. J Mass Spectrom. 1996;31:1188–90.
Hurtado PP, O’Connor PB. Differentiation of isomeric amino acid residues in protein and peptides using mass spectrometry. Mass Spectrom Rev. 2012;31:609–25.
Cooper HJ, Hakansson K, Marshall AG. The role of electron capture dissociation in biomolecular analysis. Mass Spectrom Rev. 2005;24:201–22.
Syrstad EA, Tureček F. Toward a general mechanism of electron capture dissociation. J Am Soc Mass Spectrom. 2005;16:208–24.
Fujihara A, Matsuo S, Tajiri M, Wada Y, Hayakawa S. Hypervalent radical formation probed by electron-transfer dissociation of zwitterionic tryptophan and tryptophan-containing dipeptides complexed with Ca2+ and 18-crown-6 in the gas phase. J Mass Spectrom. 2015;50:1124–9.
Qi Y, Volmer DA. Electron-based fragmentation methods in mass spectrometry: an overview. Mass Spectrom Rev. 2017;36:4–15.
Harvey DJ. Analysis of carbohydrates and glycoconjugates by matrix-assisted laser desorption/ionization mass spectrometry: an update for 2011-2012. Mass Spectrom Rev. 2017;36:255–422.
Lee HHL, Kim HI. Supramolecular analysis of monosaccharide derivatives using cucurbit[7]uril and electrospray ionization tandem mass spectrometry. Isr J Chem. 2017;57:1–8.
Sawada M. Chiral recognition detected by fast atom bombardment mass spectrometry. Mass Spectrom Rev. 1997;16:73–90.
Shizuma M, Imamura H, Takai Y, Yamada H, Takeda T, Takahashi S, et al. Facile ee-determination from a single measurement by fast atom bombardment mass spectrometry: a double labeling method. Int J Mass Spectrom. 2001;210/211:585–90.
Shizuma M, Adachi H, Kawamura M, Takai Y, Yamada H, Takeda T, et al. Chiral discrimination of fructo-oligosaccharides towards amino acid derivatives by induced-fitting chiral recognition. J Chem Soc Perkin Trans. 2001;2:592–601.
Speranza M. Enantioselectivity in gas-phase ion-molecule reactions. Int J Mass Spectrom. 2004;232:277–317.
Schug KA, Lindner W. Chiral molecular recognition for the detection and analysis of enantiomers by mass spectrometric methods. J Sep Sci. 2005;28:1932–55.
Awad H, El-Aneed A. Enantioselectivity of mass spectrometry: challenges and promises. Mass Spectrom Rev. 2013;32:466–83.
Piovesana S, Samperi R, Lagana A, Bella M. Determination of enantioselectivity and enantiomeric excess by mass spectrometry in the absence of chiral chromatographic separation: an overview. Chem Eur J. 2013;19:11478–94.
Fujihara A, Maeda N, Hayakawa S. Quantitative chiral analysis of tryptophan using enantiomer-selective photolysis of cold non-covalent complexes in the gas phase. J Mass Spectrom. 2015;50:451–3.
Fujihara A, Maeda N, Doan TN, Hayakawa S. Enantiomeric excess determination for monosaccharides using chiral transmission to cold gas-phase tryptophan in ultraviolet photodissociation. J Am Soc Mass Spectrom. 2017;28:224–8.
Fujihara A, Maeda N. Quantitative chiral analysis of amino acids in solution using enantiomer-selective photodissociation of cold gas-phase tryptophan via chiral recognition. Anal Chim Acta. 2017;979:31–5.
Yu X, Yao Z-P. Chiral recognition and determination of enantiomeric excess by mass spectrometry: a review. Anal Chim Acta. 2017;968:1–20.
Tao WA, Zhang D, Nikolaev EN, Cooks RG. Copper(II)-assisted enantiomeric analysis of d,l-amino acids using the kinetic method: chiral recognition and quantification in the gas phase. J Am Chem Soc. 2000;122:10598–609.
Yao Z-P, Wan TSM, Kwong K-P, Che C-T. Chiral analysis by electrospray ionization mass spectrometry/mass spectrometry. 2. Determination of enantiomeric excess of amino acids. Anal Chem. 2000;72:5394–401.
Tao WA, Gozzo FC, Cooks RG. Mass spectrometric quantitation of chiral drugs by the kineitc method. Anal Chem. 2001;73:1692–8.
Nagy G, Pohl NLB. Complete hexose isomer identification with mass spectrometry. J Am Soc Mass Spectrom. 2015;26:677–85.
Nagy G, Pohl NLB. Monosaccharide identification as a first step toward de novo carbohydrate sequencing: mass spectrometry strategy for the identification and differentiation of diastereomeric and enantiomeric pentose isomers. Anal Chem. 2015;87:4566–71.
Nagy G, Peng T, Pohl NLB. General label-free mass spectrometry-based assay to identify glycosidase substrate competence. Anal Chem. 2016;88:7183–90.
Bain RM, Yan X, Raab SA, Ayrton ST, Flick TG, Cooks RG. On-line chiral analysis using the kinetic method. Analyst. 2016;141:2441–6.
Domalain V, Hubert-Roux M, Tognetti V, Joubert L, Lange CM, Rouden J, et al. Enantiomeric differentiation of aromatic amino acids using traveling wave ion mobility-mass spectrometry. Chem Sci. 2014;5:3234–9.
Gaye MM, Nagy G, Clemmer DE, Pohl NLB. Multidimensional analysis of 16 glucose isomers by ion mobility spectrometry. Anal Chem. 2016;88:2335–44.
Yu X, Yao Z-P. Chiral differentiation of amino acids through binuclear copper bound tetramers by ion mobility mass spectrometry. Anal Chim Acta. 2017;981:62–70.
Fuke K, Tona M, Fujihara A, Sakurai M, Ishikawa H. Design and development of a novel nuclear magnetic resonance detection for the gas phase ions by magnetic resonance acceleration technique. Rev Sci Instrum. 2012;83:085106(1-8).
Fuke K, Ohshima Y, Tona M. Preparation of cold ions in strong magnetic field and its application to gas-phase NMR spectroscopy. Hyperfine Interact. 2015;236:9–18.
Bonner WA. The origin and amplification of biomolecular chirality. Orig Life Evol Biosph. 1991;21:59–111.
Kojo S. Origin of homochirality of amino acids in the biosphere. Symmetry. 2010;2:1022–32.
Ruiz-Mirazo K, Briones C, Escosura A. Prebiotic systems chemistry: new perspectives for the origins of life. Chem Rev. 2014;114:285–366.
Munegumi T. Aldolase as a chirality intersection of l-amino acids and d-sugars. Orig Life Evol Biosph. 2015;45:173–82.
Myrgorodska I, Meinert C, Hoffmann SV, Jones NC, Nahon L, Meierhenrich UJ. Light on chirality: absolute asymmetric formation of chiral molecules relevant in prebiotic evolution. ChemPlusChem. 2017;82:74–87.
Bernstein MP, Dworkin JP, Sandford SA, Cooper GW, Allamandola LJ. Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature. 2002;416:401–3.
Muñoz Caro GM, Meierhenrich UJ, Schutte WA, Barbier B, Segovia AA, Rosenbauer H, et al. Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature. 2002;416:403–6.
Meinert C, Myrgorodska I, Marcellus PD, Buhse T, Nahon L, Hoffmann SV, et al. Ribose and related sugars from ultraviolet irradiation of interstellar ice analogs. Science. 2016;352:208–12.
Gontareva NB, Kuzicheva EA, Shelegedin VN. Synthesis and characterization of peptides after high-energy impact on the icy matrix: preliminary step for further UV-induced formation. Planet Space Sci. 2009;57:441–5.
Kaiser RI, Stockton AM, Kim YS, Jensen EC, Mathies RA. On the formation of dipeptides in interstellar model ices. Astrophys J. 2013;765:111–9.
Abplanalp MJ, Förestel M, Kaiser RI. Exploiting single photon vacuum ultraviolet photoionization to unravel the synthesis of complex organic molecules in interstellar ices. Chem Phys Lett. 2016;644:79–98.
Cronin JR, Pizzarello S. Enantiomeric excesses in meteoritic amino acids. Science. 1997;275:951–5.
Engel MH, Macko SA. Isotopic evidence for extraterrestrial non-racemic amino acids in the Murchison meteorite. Nature. 1997;389:265–8.
Pizzarello S, Groy TL. Molecular asymmetry in extraterrestrial organic chemistry: an analytical perspective. Geochim Cosmochim Acta. 2011;75:645–56.
McGuire BA, Carroll PB, Loomis RA, Finneran IA, Jewell PR, Remijan AJ, et al. Discovery of the interstellar chiral molecule propylene oxide (CH3CHCH2O). Science. 2016;352:1449–52.
Bailey J, Chrysostomou A, Hough JH, Gledhill TM, McCall A, Clark S, et al. Circular polarization in star-formation regions: implications for biomolecular homochirality. Science. 1998;281:672–4.
Takats Z, Nanita SC, Cooks RG, Schlosser G, Vekey K. Amino acid clusters formed by sonic spray ionization. Anal Chem. 2003;75:1514–23.
Nanita SC, Cooks RG. Serine octamers: cluster formation, reactions, and implications for biomolecule homochirality. Angew Chem Int Ed. 2006;45:554–69.
Atlasevich N, Holliday AE, Valemtine SJ, Clemmer DE. Chirality and packing in small proline clusters. J Phys Chem B. 2012;116:11442–6.
Holliday AE, Atlasevich N, Myung S, Plasencia MD, Valemtine SJ, Clemmer DE. Oscillations of chiral preference in proline cluster. J Phys Chem A. 2013;117:1035–41.
Vandenbussche S, Vandenbussche G, Reisse J, Bartik K. Do serine octamers exist in solutions? Relevance of this question in the context of the origin of homochirality on earth. Eur J Org Chem. 2006;2006:3069–3073.
Fujihara A, Sato T, Hayakawa S. Enantiomer-selective ultraviolet photolysis of temperature-controlled protonated tryptophan on a chiral crown ether in the gas phase. Chem Phys Lett. 2014;610-611:228–33.
Fujihara A, Maeda N, Hayakawa S. Enantiomer-selective photolysis of cold gas-phase tryptophan in L-serine clusters with linearly polarized light. Orig Life Evol Biosph. 2014;44:67–73.
Doan TN, Fujihara A. Enantiomer-selective photo-induced reaction of protonated tryptophan with disaccharides in the gas phase. Orig Life Evol Biosph. 2018;48:123–30.
Fujihara A, Matsuyama H, Tajiri M, Wada Y, Hayakawa S. Enantioselective collision-activated dissociation of gas-phase tryptophan induced by chiral recognition of protonated l-alanine peptides. Orig Life Evol Biosph. 2017;47:161–7.
Fujihara A, Inoue H, Sogi M, Tajiri M, Wada Y. Chiral and molecular recognition through protonation between aromatic amino acids and tripeptides probed by collision-activated dissociation in the gas phase. Molecules. 2018;23:162–72.
Fujihara A, Maeda N, Hayakawa S. Chiral recognition between L-alanine peptides and tryptophan enantiomers probed by ultraviolet photodissociation in the gas phase. J Mass Spectrom. 2016;51:257–60.
Lucas B, Barat M, Fayeton JA, Perot M, Jouvet C, Grégoire G, et al. Mechanisms of photoinduced Cα–Cβ bond breakage in protonated aromatic amino acids. J Chem Phys. 2008;128:164302–8.
Grégoire G, Lucas B, Barat M, Fayeton JA, Dedonder-Lardeux C, Jouvet C. UV photoinduced dynamics in protonated aromatic amino acid. Eur Phys J D. 2009;51:109–16.
Fujihara A, Maeda N, Hayakawa S. Enantioselective photolysis and quantitative chiral analysis of tryptophan complexed with alkali-metalized l-serine in the gas phase. Chirality. 2015;27:349–52.
Grégoire G, Jouvet, Dedonder, Sobolewski AL. Ab initio study of the excited-state deactivation pathways of protonated tryptophan and tyrosine. J Am Chem Soc. 2007;129:6223–31.
Fuke K, Takasu R. Ultrafast photochemistry of ammonia clusters: formation and decay of hypervalent molecular clusters containing the NH4 radicals. Bull Chem Soc Jpn. 1995;68:3309–18.
Mercier SR, Boyarkin OV, Kamariotis A, Guglielmi M, Tavernelli I, Cascella M, et al. Microsolvation effects on the excited-state dynamics of protonated tryptophan. J Am Chem Soc. 2006;128:16938–43.
Fujihara A, Noguchi N, Yamada Y, Ishikawa H, Fuke K. Microsolvation and protonation effects on geometric and electronic structures of tryptophan and tryptophan-containing dipeptides. J Phys Chem A. 2009;113:8169–75.
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 17K14441.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Fujihara, A., Okawa, Y. Chiral and molecular recognition of monosaccharides by photoexcited tryptophan in cold gas-phase noncovalent complexes as a model for chemical evolution in interstellar molecular clouds. Anal Bioanal Chem 410, 6279–6287 (2018). https://doi.org/10.1007/s00216-018-1238-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1238-9