Abstract
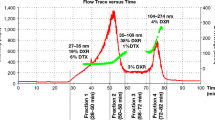
Asymmetric flow field-flow fractionation (AF4) coupled with UV-Vis spectroscopy, multi-angle light scattering (MALS) and refractive index (RI) detection has been applied for the characterization of MIL-100(Fe) nanoMOFs (metal–organic frameworks) loaded with nucleoside reverse transcriptase inhibitor (NRTI) drugs for the first time. Empty nanoMOFs and nanoMOFs loaded with azidothymidine derivatives with three different degrees of phosphorylation were examined: azidothymidine (AZT, native drug), azidothymidine monophosphate (AZT-MP), and azidothymidine triphosphate (AZT-TP). The particle size distribution and the stability of the nanoparticles when interacting with drugs have been determined in a time frame of 24 h. Main achievements include detection of aggregate formation in an early stage and monitoring nanoMOF morphological changes as indicators of their interaction with guest molecules. AF4-MALS proved to be a useful methodology to analyze nanoparticles engineered for drug delivery applications and gave fundamental data on their size distribution and stability.

ᅟ



Similar content being viewed by others
References
Furman PA, Fyfe JA, Stclair MH, Weinhold K, Rideout JL, Freeman GA, et al. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human-immunodeficiency-virus reverse-transcriptase. PNAS. 1986;83(21):8333–7.
Kukhanova M, Krayevsky A, Prusoff W, Cheng YC. Design of anti-HIV compounds: from nucleoside to nucleoside 5'-triphosphate analogs. Problems and perspectives. Curr Pharm Des. 2000;6(5):585–98.
Loke SL, Stein CA, Zhang XH, Mori K, Nakanishi M, Subasinghe C, et al. Characterization of oligonucleotide transport into living cells. PNAS. 1989;86(10):3474–8.
Li XL, Chan WK. Transport, metabolism and elimination mechanisms of anti-HIV agents. Adv Drug Deliv Rev. 1999;39(1–3):81–103.
Hillaireau H, Le Doan T, Appel M, Couvreur P. Hybrid polymer nanocapsules enhance in vitro delivery of azidothymidine-triphosphate to macrophages. J Control Release. 2006;116(3):346–52.
Hillaireau H, Le Doan T, Besnard M, Chacun H, Janin J, Couvreur P. Encapsulation of antiviral nucleotide analogues azidothymidine-triphosphate and cidofovir in poly(iso-butylcyanoacrylate) nanocapsules. Int J Pharm. 2006;324(1):37–42.
Hillaireau H, Le Doan T, Chacun H, Janin J, Couvreur P. Encapsulation of mono- and oligo-nucleotides into aqueous-core nanocapsules in presence of various water-soluble polymers. Int J Pharm. 2007;331(2):148–52.
Kohli E, Han HY, Zeman AD, Vinogradov SV. Formulations of biodegradable Nanogel carriers with 5′-triphosphates of nucleoside analogs that display a reduced cytotoxicity and enhanced drug activity. J Control Release. 2007;121(1–2):19–27.
Saiyed ZM, Gandhi NH, Nair MPNAZT. 5'-triphosphate nanoformulation suppresses human immunodeficiency virus type 1 replication in peripheral blood mononuclear cells. J Neuro-Oncol. 2009;15(4):343–7.
Saiyed ZM, Gandhi NH, Nair MPN. Magnetic nanoformulation of azidothymidine 5'-triphosphate for targeted delivery across the blood-brain barrier. Int J Nanomedicine. 2010;5:157–66.
Vinogradov SV. Polymeric nanogel formulations of nucleoside analogs. Expert Opin Drug Deliv. 2007;4(1):5–17.
Vinogradov SV, Kabanov AV. Synthesis of nanogel carriers for delivery of active phosphorylated nucleoside analogues. Abstr Pap Am Chem Soc. 2004;228:U369-U.
Gerson T, Makarov E, Senanayake TH, Gorantla S, Poluektova LY, Vinogradov SV. Nano-NRTIs demonstrate low neurotoxicity and high antiviral activity against HIV infection in the brain. Nanomedicine. 2014;10(1):177–85.
Horcajada P, Chalati T, Serre C, Gillet B, Sebrie C, Baati T, et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat Mater. 2010;9(2):172–8.
Kuppler RJ, Timmons DJ, Fang QR, Li JR, Makal TA, Young MD, et al. Potential applications of metal-organic frameworks. Coord Chem Rev. 2009;253(23–24):3042–66.
Della Rocca J, Liu DM, Lin WB. Nanoscale metal-organic frameworks for biomedical imaging and drug delivery. Acc Chem Res. 2011;44(10):957–68.
Horcajada P, Gref R, Baati T, Allan PK, Maurin G, Couvreur P, et al. Metal-organic frameworks in biomedicine. Chem Rev. 2012;112(2):1232–68.
Simon-Yarza MT, Baati T, Paci A, Lesueur LL, Seck A, Chiper M, et al. Antineoplastic busulfan encapsulated in a metal organic framework nanocarrier: first in vivo results. J Mater Chem B. 2016;4(4):585–8.
Chalati T, Horcajada P, Couvreur P, Serre C, Ben Yahia M, Maurin G, et al. Porous metal organic framework nanoparticles to address the challenges related to busulfan encapsulation. Nanomedicine. 2011;6(10):1683–95.
Horcajada P, Serre C, McKinlay AC, Morris RE. Biomedical applications of metal-organic frameworks. Metal-organic frameworks: applications from catalysis to gas storage 2011. p. 215–50.
Anand R, Borghi F, Manoli F, Manet I, Agostoni V, Reschiglian P, et al. Host-guest interactions in Fe(III)-trimesate MOF nanoparticles loaded with doxorubicin. J Phys Chem B. 2014;118(29):8532–9.
Agostoni V, Anand R, Monti S, Hall S, Maurin G, Horcajada P, et al. Impact of phosphorylation on the encapsulation of nucleoside analogues within porous iron(III) metal-organic framework MIL-100(Fe) nanoparticles. J Mater Chem B. 2013;1(34):4231–42.
Simon-Yarza T, Giménez-Marqués M, Mrimi R, Mielcarek A, Gref R, Horcajada P, et al. A smart metal-organic framework nanomaterial for lung targeting. Angew Chem Int Ed Engl. 2017;56(49):15565–9.
Rodriguez-Ruiz V, Maksimenko A, Anand R, Monti S, Agostoni V, Couvreur P, et al. Efficient “green” encapsulation of a highly hydrophilic anticancer drug in metal-organic framework nanoparticles. J Drug Target. 2015;23(7–8):759–67.
Horcajada P, Surble S, Serre C, Hong DY, Seo YK, Chang JS, et al. Synthesis and catalytic properties of MIL-100(Fe), an iron(III) carboxylate with large pores. Chem Commun. 2007 (27):2820–2.
Baati T, Njim L, Neffati F, Kerkeni A, Bouttemi M, Gref R, et al. In depth analysis of the in vivo toxicity of nanoparticles of porous iron(III) metal-organic frameworks. Chem Sci. 2013;4(4):1597–607.
Brar SK, Verma M. Measurement of nanoparticles by light-scattering techniques. TrAC Trends Anal Chem. 2011;30(1):4–17.
Kaasalainen M, Aseyev V, von Haartman E, Karaman DŞ, Mäkilä E, Tenhu H, et al. Size, stability, and porosity of mesoporous nanoparticles characterized with light scattering. Nanoscale Res Lett. 2017;12(1):74.
Giddings JC. Field-flow fractionation: analysis of macromolecular, colloidal, and particulate materials. Science. 1993;260(5113):1456.
Contado C. Field flow fractionation techniques to explore the “nano-world”. Anal Bioanal Chem. 2017;409(10):2501–18.
Rigaux G, Gheran CV, Callewaert M, Cadiou C, Voicu SN, Dinischiotu A, et al. Characterization of Gd loaded chitosan-TPP nanohydrogels by a multi-technique approach combining dynamic light scattering (DLS), asymetrical flow-field-flow-fractionation (AF4) and atomic force microscopy (AFM) and design of positive contrast agents for molecular resonance imaging (MRI). Nanotechnology. 2017;28(5):055705.
Schimpf ME, Caldwell K, Giddings JC. Field-flow fractionation handbook. Hoboken: Wiley; 2000.
Wyatt PJ. Light-scattering and the absolute characterization of macromolecules. Anal Chim Acta. 1993;272(1):1–40.
Thielking H, Roessner D, Kulicke W-M. Online coupling of flow field-flow fractionation and multiangle laser light scattering for the characterization of polystyrene particles. Anal Chem. 1995;67(18):3229–33.
Wyatt PJ. Submicrometer particle sizing by multiangle light scattering following fractionation. J Colloid Interface Sci. 1998;197(1):9–20.
Reschiglian P, Rambaldi DC, Zattoni A. Flow field-flow fractionation with multiangle light scattering detection for the analysis and characterization of functional nanoparticles. Anal Bioanal Chem. 2011;399(1):197–203.
Zattoni A, Rambaldi DC, Reschiglian P, Melucci M, Krol S, Garcia AMC, et al. Asymmetrical flow field-flow fractionation with multi-angle light scattering detection for the analysis of structured nanoparticles. J Chromatogr A. 2009;1216(52):9106–12.
Marassi V, Roda B, Zattoni A, Tanase M, Reschiglian P. Hollow fiber flow field-flow fractionation and size-exclusion chromatography with MALS detection: a complementary approach in biopharmaceutical industry. J Chromatogr A. 2014;1372C:196–203.
Hupfeld S, Moen HH, Ausbacher D, Haas H, Brandl M. Liposome fractionation and size analysis by asymmetrical flow field-flow fractionation/multi-angle light scattering: influence of ionic strength and osmotic pressure of the carrier liquid. Chem Phys Lipids. 2010;163(2):141–7.
Kaluderovic GN, Dietrich A, Kommera H, Kuntsche J, Mader K, Mueller T, et al. Liposomes as vehicles for water insoluble platinum-based potential drug: 2-(4-(tetrahydro-2H-pyran-2-yloxy)-undecyl)-propane-1,3-diamminedichloro platinum(II). Eur J Med Chem. 2012;54:567–72.
Kang DY, Kim MJ, Kim ST, Oh KS, Yuk SH, Lee SH. Size characterization of drug-loaded polymeric core/shell nanoparticles using asymmetrical flow field-flow fractionation. Anal Bioanal Chem. 2008;390(8):2183–8.
Gaulding JC, South AB, Lyon LA. Hydrolytically degradable shells on thermoresponsive microgels. Colloid Polym Sci. 2013;291(1):99–107.
Schadlich A, Caysa H, Mueller T, Tenambergen F, Rose C, Gopferich A, et al. Tumor accumulation of NIR fluorescent PEG PLA nanoparticles: impact of particle size and human xenograft tumor model. ACS Nano. 2011;5(11):8710–20.
Schadlich A, Rose C, Kuntsche J, Caysa H, Mueller T, Gopferich A, et al. How stealthy are PEG-PLA nanoparticles? An NIR in vivo study combined with detailed size measurements. Pharm Res. 2011;28(8):1995–2007.
Shimoda A, Sawada S, Kano A, Maruyama A, Moquin A, Winnik FM, et al. Dual crosslinked hydrogel nanoparticles by nanogel bottom-up method for sustained-release delivery. Colloids Surf B Biointerfaces. 2012;99:38–44.
Ma PL, Buschmann MD, Winnik FM. One-step analysis of DNA/chitosan complexes by field-flow fractionation reveals particle size and free chitosan content. Biomacromolecules. 2010;11(3):549–54.
Augsten C, Mader K. Characterizing molar mass distributions and molecule structures of different chitosans using asymmetrical flow field-flow fractionation combined with multi-angle light scattering. Int J Pharm. 2008;351(1–2):23–30.
Pease LF, Lipin DI, Tsai DH, Zachariah MR, Lua LHL, Tarlov MJ, et al. Quantitative characterization of virus-like particles by asymmetrical flow field flow fractionation, electrospray differential mobility analysis, and transmission electron microscopy. Biotechnol Bioeng. 2009;102(3):845–55.
Chuan YP, Fan YY, Lua L, Middelberg APJ. Quantitative analysis of virus-like particle size and distribution by field-flow fractionation. Biotechnol Bioeng. 2008;99(6):1425–33.
Zillies JC, Zwiorek K, Winter G, Coester C. Method for quantifying the PEGylation of gelatin nanoparticle drug carrier systems using asymmetrical flow field-flow fractionation and refractive index detection. Anal Chem. 2007;79(12):4574–80.
Garcea RL, Gissmann L. Virus-like particles as vaccines and vessels for the delivery of small molecules. Curr Opin Biotechnol. 2004;15(6):513–7.
Fraunhofer W, Winter G, Coester C. Asymmetrical flow field-flow fractionation and multiangle light scattering for analysis of gelatin nanoparticle drug carrier systems. Anal Chem. 2004;76(7):1909–20.
Agostoni V, Horcajada P, Rodriguez-Ruiz V, Willaime H, Couvreur P. Serre C, et al. ‘Green’ fluorine-free mesoporous iron(III) trimesate nanoparticles for drug delivery. Green Mat. 2013;1(4):209–17.
Marassi V, Roda B, Casolari S, Ortelli S, Blosi M, Zattoni A, et al. Hollow-fiber flow field-flow fractionation and multi-angle light scattering as a new analytical solution for quality control in pharmaceutical nanotechnology. Microchem J. 2018;136:149–56.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Andrea Zattoni, Barbara Roda, and Pierluigi Reschiglian are associates of the academic spinoff company byFlow Srl (Bologna, Italy). The company mission includes know-how transfer, development, and application of novel technologies and methodologies for the analysis and characterization of samples of nanobiotechnological interest. Valentina Marassi, Francesco Borghi, Resmi Anand, Valentina Agostoni, Ruxandra Gref, and Sandra Monti declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Roda, B., Marassi, V., Zattoni, A. et al. Flow field-flow fractionation and multi-angle light scattering as a powerful tool for the characterization and stability evaluation of drug-loaded metal–organic framework nanoparticles. Anal Bioanal Chem 410, 5245–5253 (2018). https://doi.org/10.1007/s00216-018-1176-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1176-6