Abstract
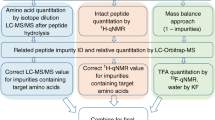
Peptides are an increasingly important group of biomarkers and pharmaceuticals. The accurate purity characterization of peptide calibrators is critical for the development of reference measurement systems for laboratory medicine and quality control of pharmaceuticals. The peptides used for these purposes are increasingly produced through peptide synthesis. Various approaches (for example mass balance, amino acid analysis, qNMR, and nitrogen determination) can be applied to accurately value assign the purity of peptide calibrators. However, all purity assessment approaches require a correction for structurally related peptide impurities in order to avoid biases. Liquid chromatography coupled to high resolution mass spectrometry (LC-hrMS) has become the key technique for the identification and accurate quantification of structurally related peptide impurities in intact peptide calibrator materials. In this study, LC-hrMS-based methods were developed and validated in-house for the identification and quantification of structurally related peptide impurities in a synthetic human C-peptide (hCP) material, which served as a study material for an international comparison looking at the competencies of laboratories to perform peptide purity mass fraction assignments. More than 65 impurities were identified, confirmed, and accurately quantified by using LC-hrMS. The total mass fraction of all structurally related peptide impurities in the hCP study material was estimated to be 83.3 mg/g with an associated expanded uncertainty of 3.0 mg/g (k = 2). The calibration hierarchy concept used for the quantification of individual impurities is described in detail.

ᅟ




Similar content being viewed by others
Abbreviations
- CCQM:
-
Consultative Committee for Amount of Substance: Metrology in Chemistry and Biology
- CID:
-
Collision induced dissociation
- ESI:
-
Electrospray ionization
- HCD:
-
High energy collision dissociation
- hCP:
-
Human C-peptide
- hrMS:
-
High resolution mass spectrometry
- IDMS:
-
Isotope dilution tandem mass spectrometry
- LC:
-
Liquid chromatography
- MS:
-
Mass spectrometry
- PAWG:
-
Protein Analysis Working Group
- PICAA:
-
Peptide impurity corrected amino acid analysis
- qNMR:
-
Quantitative nuclear magnetic resonance spectrometry
References
Kjems LL, Vølund A, Madsbad S. Quantification of beta-cell function during IVGTT in type II and non-diabetic subjects: assessment of insulin secretion by mathematical methods. Diabetologia. 2001;44(10):1339–48.
Polonsky KS, Licinio-Paixao J, Given BD, Pugh W, Rue P, Galloway J, et al. Use of biosynthetic human C-peptide in the measurement of insulin secretion rates in normal volunteers and type I diabetic patients. J Clin Invest. 1986;77(1):98–105.
Little RR, Rohlfing CL, Tennill AL, Madsen RW, Polonsky KS, Myers GL, et al. Standardization of C-peptide measurements. Clin Chem. 2008;54(6):1023–6.
Fierens C, Stöckl D, Baetens D, De Leenheer AP, Thienpont LM. Application of a C-peptide electrospray ionization-isotope dilution–liquid chromatography-tandem mass spectrometry measurement procedure for the evaluation of five C-peptide immunoassays for urine. J Chromatogr B. 2003;792(2):249–59.
Rodríguez-Cabaleiro D, Stöckl D, Thienpont LM. Improvement of sample pretreatment prior to analysis of C-peptide in serum by isotope-dilution liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2005;19(23):3600–2.
Rodríguez Cabaleiro D, Stöckl D, Kaufman JM, Fiers T, Thienpont LM. Feasibility of standardization of serum C-peptide immunoassays with isotope-dilution liquid chromatography-tandem mass spectrometry. Clin Chem. 2006;52(6):1193–6.
Vesper HW, Thienpont LM. Traceability in laboratory medicine. Clin Chem. 2009;55(6):1067–75.
Bristow AF, Das RE. WHO international reference reagents for human proinsulin and human insulin C-peptide. J Biol Stand. 1988;16(3):179–86.
Josephs RD, Stoppacher N, Daireaux A, Choteau T, Lippa KA, Phinney KW, et al. State-of-the-art and trends for the SI traceable value assignment of the purity of peptides using the model compound angiotensin I. Trends Anal Chem. 2018;101:108–19.
Westwood S, Choteau T, Daireaux A, Josephs RD, Wielgosz RI. Mass balance method for the SI value assignment of the purity of organic compounds. Anal Chem. 2013;85(6):3118–26.
Stoppacher N, Josephs RD, Daireaux A, Choteau T, Westwood SW, Wielgosz RI. Impurity identification and determination for the peptide hormone angiotensin I by liquid chromatography-high-resolution tandem mass spectrometry and the metrological impact on value assignments by amino acid analysis. Anal Bioanal Chem. 2013;405(25):8039–51.
Stoppacher N, Josephs RD, Daireaux A, Choteau T, Westwood S, Wielgosz RI. Accurate quantification of impurities in pure peptide material—angiotensin I: comparison of calibration requirements and method performance characteristics of liquid chromatography coupled to hybrid tandem mass spectrometry and linear ion trap high-resolution mass spectrometry. Rapid Commun Mass Spectrom. 2015;29(18):1651–60.
University of California. MS-Product. 2013; http://prospector.ucsf.edu/prospector/cgi-bin/msform.cgi?form=msproduct. Accessed last on 16 Feb 2018.
Kinumi T, Goto M, Eyama S, Kato M, Kasama T, Takatsu A. Development of SI-traceable C-peptide certified reference material NMIJ CRM 6901-a using isotope-dilution mass spectrometry-based amino acid analyses. Anal Bioanal Chem. 2012;404(1):13–21.
Sanz-Nebot V, Benavente F, Barbosa J. Separation and characterization of multicomponent peptide mixtures by liquid chromatography–electrospray ionization mass spectrometry: application to crude products of the synthesis of leuprolide. J Chromatogr A. 2000;870(1–2):315–34.
Bros P, Josephs RD, Stoppacher N, Cazals G, Lehmann S, Hirtz C, et al. Impurity determination for hepcidin by liquid chromatography-high resolution and ion mobility mass spectrometry for the value assignment of candidate primary calibrators. Anal Bioanal Chem. 2017;409(10):2559–67.
Josephs RD, Stoppacher N, Westwood S, Wielgosz RI, Li M, Quaglia M, et al. Concept paper on SI value assignment of purity—model for the classification of peptide/protein purity determinations. J Chem Metrol. 2017;11(1):1–8.
Josephs RD, Li M, Song D, Daireaux A, Choteau T, Stoppacher N, et al. Key comparison study on peptide purity-synthetic human C-peptide. Metrologia. 2017;54(Technical Supplement,1A):08007.
Josephs RD, Li M, Song D, Daireaux A, Choteau T, Stoppacher N, et al. Pilot study on peptide purity-synthetic human C-peptide. Metrologia. 2017;54(Technical Supplement,1A):080011.
Little RR, Wielgosz RI, Josephs RD, Kinumi T, Takatsu A, Li H, et al. Implementing a reference measurement system for C-peptide: successes and lessons learned. Clin Chem. 2017;63(9):1447–56.
Funding
The authors would like to thank the Ministry of Science and Technology of China for funding support (No. 2014DFA31810).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiments were conducted with synthetic hCP materials provided by the National Institute of Metrology of China commercially sourced from GenScript (China).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 1287 kb)
Rights and permissions
About this article
Cite this article
Li, M., Josephs, R.D., Daireaux, A. et al. Identification and accurate quantification of structurally related peptide impurities in synthetic human C-peptide by liquid chromatography–high resolution mass spectrometry. Anal Bioanal Chem 410, 5059–5070 (2018). https://doi.org/10.1007/s00216-018-1155-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1155-y