Abstract
In Part I of the present review series, I presented the current state of the water environment by focusing on Japanese cases and discussed the need to further develop microbial biosensor technologies for the actual water environment. I comprehensively present trends after approximately 2010 in microbial biosensor development for the water environment. In the first section, after briefly summarizing historical studies, recent studies on microbial biosensor principles are introduced. In the second section, recent application studies for the water environment are also introduced. Finally, I conclude the present review series by describing the need to further develop microbial biosensor technologies.

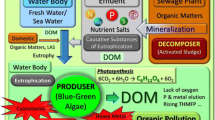
Current water pollution indirectly occurs by anthropogenic eutrophication (Part I). Recent trends in microbial biosensor development for water environment are described in part II of the present review series.
Similar content being viewed by others
Abbreviations
- BL:
-
Bioluminescence
- BOD:
-
Biochemical oxygen demand
- C. albicans :
-
Candida albicans
- C. butyricum :
-
Clostridium butyricum
- C. violaceum :
-
Chromobacterium violaceum
- CL:
-
Chemiluminescence
- CNT:
-
Carbon nanotube
- COD:
-
Chemical oxygen demand
- DCIP:
-
2,6-Dichlorophenolindophenol
- DM:
-
Double mediator
- DO:
-
Dissolved oxygen
- DOM:
-
Dissolved organic matter
- E. coli :
-
Escherichia coli
- FCN:
-
Ferricyanide
- FIA:
-
Flow injection analysis
- FL:
-
Fluorescence
- GFP:
-
Green fluorescent protein
- LASs:
-
Linear alkylbenzene sulfonates
- M. aeruginosa :
-
Microcystis aeruginosa
- MDC:
-
Microbial desalination cell
- MEMS:
-
Microelectromechanical system
- MFC:
-
Microbial fuel cell
- MWCNT:
-
Multiwall carbon nanotube
- N:
-
Nitrogen
- NP:
-
Nonylphenol
- P:
-
Phosphorus
- P. agardhii :
-
Planktothrix agardhii
- P. aeruginosa :
-
Pseudomonas aeruginosa
- P. fluorescens :
-
Pseudomonas fluorescens
- RCI:
-
Redox color indicator
- S. cerevisiae :
-
Saccharomyces cerevisiae
- SM:
-
Single mediator
- SOM:
-
Sediment organic matter
- TN:
-
Total nitrogen
- T. cutaneum :
-
Trichosporon cutaneum
- TP:
-
Total phosphorus
References
Updike SJ, Hicks GP. The enzyme electrode. Nature. 1967;214:986–8. https://doi.org/10.1038/214986a0.
Scheller FW, Wollenberger U, Warsinke A, Lisdat F. Research and development in biosensors. Curr Opin Biotechnol. 2001;12:35–40. https://doi.org/10.1016/S0958-1669(00)00169-5.
Nakamura H, Karube I. Current research activity in biosensors. Anal Bioanal Chem. 2003;377:446–68. https://doi.org/10.1007/s00216-003-1947-5.
Lee TMH. Over-the-counter biosensors: past, present, and future. Sensors. 2008;8:5535–59. https://doi.org/10.3390/s8095535.
Goode JA, Rushworth JVH, Millner PA. Biosensor regeneration: a review of common techniques and outcomes. Langmuir. 2014;31:6267–76. https://doi.org/10.1021/la503533g.
Scheller FW, Yarman A, Bachmann T, Hirsch T, Kubick S, Renneberg R, et al. Future of biosensors: a personal view. Adv Biochem Eng Biotechnol. 2014;140:1–28. https://doi.org/10.1007/10_2013_251.
Vigneshvar S, Sudhakumari CC, Senthilkumaran B, Prakash H. Recent advances in biosensor technology for potential applications – an overview. Front Bioeng Biotechnol. 2016;4:11. https://doi.org/10.3389/fbioe.2016.00011.
Turner APF. Biosensors: sense and sensibility. Chem Soc Rev. 2013;42:3184–96. https://doi.org/10.1039/C3CS35528D.
Gruhl FJ, Rapp BE, Lange K. Biosensors for diagnostic applications. Adv Biochem Eng Biotechnol. 2013;133:115–48. https://doi.org/10.1007/10_2011_130.
Gonchar M, Smutok O, Karkovska M, Stasyuk N, Gayda G. Yeast-based biosensors for clinical diagnostics and food control. In: Sibirny AA, editor. Biotechnology of yeasts and filamentous fungi. Berlin: Springer; 2017. p. 391–412.
Thakur MS, Ragavan KV. Biosensors in food processing. J Food Sci Technol. 2013;50:625–41. https://doi.org/10.1007/s13197-012-0783-z.
Dorst BV, Mehta J, Bekaert K, Rouah-Martin E, Coen WD, Dubruel P, et al. Recent advances in recognition elements of food and environmental biosensors: a review. Biosens Bioelectron. 2010;26:1178–94. https://doi.org/10.1016/j.bios.2010.07.033.
Rogers KR. Recent advances in biosensor techniques for environmental monitoring. Anal Chim Acta. 2006;568:222–31. https://doi.org/10.1016/j.aca.2005.12.067.
Bahadır EB, Sezgintürk MK. Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal Biochem. 2015;478:107–20. https://doi.org/10.1016/j.ab.2015.03.011.
Karube I, Matsunaga T, Mitsuda S, Suzuki S. Microbial electrode BOD sensor. Biotechnol Bioeng. 1977;19:1535–47. https://doi.org/10.1002/bit.260191010.
Thouand G. Microorganisms for analysis. Anal Bioanal Chem. 2011;400:893–4. https://doi.org/10.1007/s00216-011-4843-4.
Nakamura H, Karube I. Microbial Biosensors. In: Grimes CA, Pishko DV, Pishko MV, editors. Encyclopedia of sensors, vol. 6. California: American Scientific Publishers; 2005. p. 87–126. http://www.aspbs.com/eos/.
Nakamura H, Shimomura-Shimizu M, Karube I. Development of microbial sensors and their application. Adv Biochem Eng Biotechnol. 2008;109:351–94. https://doi.org/10.1007/978-3-540-75201-1.
D’Souza SF. Microbial biosensors. Biosens Bioelectron. 2001;16:337–53. https://doi.org/10.1016/S0956-5663(01)00125-7.
Lei Y, Chen W, Mulchandani A. Microbial biosensors. Anal Chim Acta. 2006;568:200–10. https://doi.org/10.1016/j.aca.2005.11.065.
Nakamura H. Recent organic pollution and its biosensing methods. Anal Methods. 2010;2:430–44. https://doi.org/10.1039/b9ay00315k.
Su L, Jia W, Hou C, Lei Y. Microbial biosensors: a review. Biosens Bioelectron. 2011;26:1788–99. https://doi.org/10.1016/j.bios.2010.09.005.
Eltzov E, Marks RS. Whole-cell aquatic biosensors. Anal Bioanal Chem. 2011;400:895–913. https://doi.org/10.1007/s00216-010-4084-y.
Abrevaya XC, Sacco NJ, Bonetto MC, Hilding-Ohlsson A, Cortón E. Analytical applications of microbial fuel cells. Part I: Biochemical oxygen demand. Biosens Bioelectron. 2015;63:580–90. https://doi.org/10.1016/j.bios.2014.04.034.
Abrevaya XC, Sacco NJ, Bonetto MC, Hilding-Ohlsson A, Cortón E. Analytical applications of microbial fuel cells. Part II: Toxicity, microbial activity and quantification, single analyte detection, and other uses. Biosens Bioelectron. 2015;63:591–601. https://doi.org/10.1016/j.bios.2014.04.053.
Yang H, Zhou M, Liu M, Yang W, Gu T. Microbial fuel cells for biosensor applications. Biotechnol Lett. 2015;37:2357–64. https://doi.org/10.1007/s10529-015-1929-7.
Hikuma M, Suzuki H, Yasuda T, Karube I, Suzuki S. Amperometric estimation of BOD by using living immobilized yeasts. Eur J Appl Microbiol Biotechnol. 1979;8:289–97. https://doi.org/10.1007/BF00508793.
Adeniran A, Sherer M, Tyo KEJ. Yeast-based biosensors: design and applications. FEMS Yeast Res. 2015;15:1–15. https://doi.org/10.1111/1567-1364.12203.
Jarque S, Bittner M, Blaha L, Hilscherova K. Yeast biosensors for detection of environmental pollutants: current state and limitations. Trends Biotechnol. 2016;34:408–19. https://doi.org/10.1016/j.tibtech.2016.01.007.
Pasco N, Baronian KHR, Jeffries C, Hay J. Biochemical mediator demand – a novel rapid alternative for measuring biochemical oxygen demand. Appl Microbiol Biotechnol. 2000;53:613–8. https://doi.org/10.1007/s002530051.
Yoshida N, Yano K, Morita T, McNiven SJ, Nakamura H, Karube I. A mediator-type biosensor as a new approach to biochemical oxygen demand estimation. Analyst. 2000;125:2280–4. https://doi.org/10.1039/b005995l.
O’Reilly JE. Oxidation-reduction potential of the ferro-ferricyanide system in buffer solutions. Biochim Biophys Acta Bioenerg. 1973;292:509–15. https://doi.org/10.1016/0005-2728(73)90001-7.
Yoshida N, Hoashi J, Morita T, McNiven SJ, Yano K, Yoshida A, et al. Monitoring of composting process using a mediator-type biochemical oxygen demand sensor. Analyst. 2001;126:1751–5. https://doi.org/10.1039/B103215C.
Yoshida N, Hoashi J, Morita T, McNiven SJ, Nakamura H, Karube I. Improvement of a mediator-type biochemical oxygen demand sensor for on-site measurement. J Biotechnol. 2001;88:269–75. https://doi.org/10.1016/S0168-1656(01)00282-6.
Yashiki Y, Yamashoji S. Extracellular reduction of menadione and ferricyanide in yeast cell suspension. J Ferment Bioeng. 1996;82:319–21. https://doi.org/10.1016/0922-338X(96)88828-3.
Baronian KHR, Downard AJ, Lowen RK, Pasco N. Detection of two distinct substrate-dependent catabolic responses in yeast cells using a mediated electrochemical method. Appl Microbiol Biotechnol. 2002;60:108–13. https://doi.org/10.1007/s00253-002-1108-3.
Yamashoji S, Yoshikawa N, Kirihara M, Tsuneyoshi T. Screening test for rapid food safety evaluation by menadione-catalysed chemiluminescent assay. Food Chem. 2013;138:2146–51. https://doi.org/10.1016/j.foodchem.2012.12.037.
Yamashoji S. Synergistic reduction of toluylene blue induced by acetaldehyde and menadione in yeast cell suspension: application to determination of yeast cell activity. Biochem Biophys Rep. 2017;9:335–40. https://doi.org/10.1016/j.bbrep.2017.01.015.
Chelikani V, Rawson FJ, Downard AJ, Gooneratne R, Kunze G, Pasco N, et al. Electrochemical detection of oestrogen binding protein interaction with estrogen in Candida albicans cell lysate. Biosens Bioelectron. 2011;26:3737–41. https://doi.org/10.1016/j.bios.2011.01.016.
Chelikani V, Downard AJ, Kunze G, Gooneratne R, Pasco N, Baronian KHR. Investigating yeast cell responses to oestrogen by electrochemical detection. Electrochim Acta. 2012;73:136–40. https://doi.org/10.1016/j.electacta.2011.11.078.
Vijayan V, Giersberg M, Chamas A, Mehrotra M, Chelikani V, Kunze G, et al. Use of recombinant estrogen binding protein for the electrochemical detection of oestrogen. Biosens Bioelectron. 2015;66:379–84. https://doi.org/10.1016/j.bios.2014.11.043.
Nakamura H, Suzuki K, Ishikuro H, Kinoshita S, Koizumi R, Okuma S, et al. A new BOD estimation method employing a double-mediator system by ferricyanide and menadione using the eukaryote Saccharomyces cerevisiae. Talanta. 2007;72:210–6. https://doi.org/10.1016/j.talanta.2006.10.019.
Nakamura H, Suzuki K, Okuma S, Yataka M, Mogi Y, Karube I. Improvement of double mediator system for BOD determination. Res Rev ElectroChem. 2008;1:21–5.
Nakamura H, Tanaka R, Suzuki K, Yataka M, Mogi Y. A direct determination method for ethanol concentrations in alcoholic beverages employing a eukaryote double-mediator system. Food Chem. 2009;117:509–13. https://doi.org/10.1016/j.foodchem.2009.04.026.
Pasco NF, Weld RJ, Hay JM, Gooneratne R. Development and applications of whole cell biosensors for ecotoxicity testing. Anal Bioanal Chem. 2011;400:931–45. https://doi.org/10.1007/s00216-011-4663-6.
Lagarde F, Jaffrezic-Renault N. Cell-based electrochemical biosensors for water quality assessment. Anal Bioanal Chem. 2011;40:947–64. https://doi.org/10.1007/s00216-011-4816-7.
Nakamura H, Abe Y, Koizumi R, Suzuki K, Mogi Y, Hirayama T, et al. A chemiluminescence biochemical oxygen demand measuring method. Anal Chim Acta. 2007;602:94–100. https://doi.org/10.1016/j.aca.2007.08.050.
Nakamura H, Hasegawa M, Nomura Y, Arikawa Y, Matsukawa R, Ikebukuro K, et al. Development of a highly sensitive chemiluminescence flow-injection analysis sensor for phosphate-ion detection using maltose phosphorylase. J Biotechnol. 1999;75:127–33. https://doi.org/10.1016/S0168-1656(99)00150-9.
Nakamura H, Tanaka H, Hasegawa M, Masuda Y, Arikawa Y, Nomura Y, et al. An automatic flow-injection analysis system for determining phosphate ion in river water using pyruvate oxidase G (from Aerococcus viridans). Talanta. 1999;50:799–807. https://doi.org/10.1016/S0039-9140(99)00137-X.
Nakamura H, Yamazaki R, Shirai T, Sano H, Nakami Y, Ikebukuro K, et al. Development of an enzymatic flow-injection chemiluminescence system for determining inorganic pyrophosphate ion. Anal Chim Acta. 2004;518:45–9. https://doi.org/10.1016/j.aca.2004.05.057.
Li J, Yu Y, Wang Y, Qian J, Zhi J. The benzoquinone-mediated electrochemical microbial biosensor for water biotoxicity assay. Electrochim Acta. 2013;97:52–7. https://doi.org/10.1016/j.electacta.2013.02.071.
Gao G, Qian J, Fang D, Yu Y, Zhi J. Development of a mediated whole cell-based electrochemical biosensor for joint toxicity assessment of multi-pollutants using a mixed microbial consortium. Anal Chim Acta. 2016;924:21–8. https://doi.org/10.1016/j.aca.2016.04.011.
Khor BH, Ismail AK, Ahamad R, Shahir S. A redox mediated UME biosensor using immobilized Chromobacterium violaceum strain R1 for rapid biochemical oxygen demand measurement. Electrochim Acta. 2015;176:777–83. https://doi.org/10.1016/j.electacta.2015.07.089.
Hassan RY, Bilitewski U. A viability assay for Candida albicans based on the electron transfer mediator 2,6-dichlorophenolindophenol. Anal Biochem. 2011;419:26–32. https://doi.org/10.1016/j.ab.2011.07.025.
Hassan RY, Bilitewski U. Direct electrochemical determination of Candida albicans activity. Biosens Bioelectron. 2013;49:192–8. https://doi.org/10.1016/j.bios.2013.05.015.
Demirkol DO, Timur S. Chitosan matrices modified with carbon nanotubes for use in mediated microbial biosensing. Microchim Acta. 2011;173:537–42. https://doi.org/10.1007/s00604-011-0596-1.
Šefčovičová J, Filip J, Gemeiner P, Vikartovská A, Tkac J. High performance microbial 3-D bionanocomposite as a bioanode for a mediated biosensor device. Electrochem Commun. 2011;13:966–8. https://doi.org/10.1016/j.elecom.2011.06.013.
Hassan RYA, Wollenberger U. Mediated bioelectrochemical system for biosensing the cell viability of Staphylococcus aureus. Anal Bioanal Chem. 2016;408:579–87. https://doi.org/10.1007/s00216-015-9134-z.
Hassan RYA, Hassan HNA, Abdel-Aziz MS, Khaled E. Nanomaterials-based microbial sensor for direct electrochemical detection of Streptomyces Spp. Sensors Actuators B Chem. 2014;203:848–53. https://doi.org/10.1016/j.snb.2014.07.059.
Sedki M, Hassan RYA, Hefnawy A, El-Sherbiny IM. Sensing of bacterial cell viability using nanostructured bioelectrochemical system: rGO-hyperbranched chitosan nanocomposite as a novel microbial sensor platform. Sensors Actuators B Chem. 2017;252:191–200. https://doi.org/10.1016/j.snb.2017.05.163.
Kang YO, Choi SH, Gopalan A, Lee KP, Kang HD, Song YS. One pot synthesis of a few nanocomposites with poly(N-vinylcarbazole) and CdS, Ag, Pd50-Ag50, and Pt50-Ru50 nanoparticles using γ-irradiation. J Appl Polym Sci. 2006;100:1809–15. https://doi.org/10.1002/app.23078.
Kim SK, Kwen HD, Choi SH. Fabrication of a microbial biosensor based on QD-MWNT supports by a one-step radiation reaction and detection of phenolic compounds in red wines. Sensors. 2011;11:2001–12. https://doi.org/10.3390/s110202001.
Anu Prathap MU, Chaurasia AK, Sawant SN, Apte SK. Polyaniline-based highly sensitive microbial biosensor for selective detection of lindane. Anal Chem. 2012;84:6672–8. https://doi.org/10.1021/ac301077d.
Mittal SK, Singh J, Kumar SK. Chlorella modified glassy carbon electrode as whole cell microbial sensor for heavy metal ions. Curr Anal Chem. 2012;8:365–72. https://doi.org/10.2174/157341112801264932.
Nakamura H, Kobayashi S, Hirata Y, Suzuki K, Mogi Y, Karube I. A spectrophotometric biochemical oxygen demand determination method using 2,6-dichlorophenolindophenol as the redox color indicator and the eukaryote Saccharomyces cerevisiae. Anal Biochem. 2007;369:168–74. https://doi.org/10.1016/j.ab.2007.06.040.
Yoshida N, McNiven SJ, Morita T, Nakamura H, Karube I. A simple, multiple simultaneous spectrophotometric method for BOD determination using DCIP as the redox color indicator. Anal Lett. 2002;35:1541–9. https://doi.org/10.1081/AL-120006729.
Yoshida N, McNiven SJ, Yoshida A, Morita T, Nakamura H, Karube I. A compact optical system for multi-determination of biochemical oxygen demand using disposable strips. Field Anal Chem Technol. 2001;5:222–7. https://doi.org/10.1002/fact.10001.
Nakamura H, Mogi Y, Hattori H, Kita Y, Hattori D, Yoshimura A, et al. Absorption-based highly sensitive and reproducible biochemical oxygen demand measurement method for seawater using salt-tolerant yeast Saccharomyces cerevisiae ARIF KD-003. Anal Chim Acta. 2008;620:127–33. https://doi.org/10.1016/j.aca.2008.05.008.
Nakamura H, Hirata Y, Mogi Y, Kobayashi S, Suzuki K, Hirayama T, et al. A simple and highly repeatable colorimetric toxicity assay method using DCIP as the redox color indicator and whole eukaryote cells. Anal Bioanal Chem. 2007;389:835–40. https://doi.org/10.1007/s00216-007-1527-1.
Nakamura H, Hattori D, Tokunaga D, Suzuki Y. An isothermal absorptiometric assay for viable microbes using redox color indicator 2,6-dichlorophenolindophenol. Anal Biochem. 2013;441:140–6. https://doi.org/10.1016/j.ab.2013.07.010.
Zhai J, Liu L, Yong D, Li D, Dong S. Neutral red based colorimetric microorganism bioassay for direct toxicity assessment of toxic chemicals in water. Anal Methods. 2012;4:3849–54. https://doi.org/10.1039/C2AY25899D.
Zhai J, Yong D, Li J, Dong S. A novel colorimetric biosensor for monitoring and detecting acute toxicity in water. Analyst. 2013;138:702–7. https://doi.org/10.1039/C2AN36160D.
Nakamura H, Suzuki M. New concept for a toxicity assay based on multiple indexes from the wave shape of damped metabolic oscillation induced in living yeast cells – Part I: Characterization of the phenomenon. Anal Bioanal Chem. 2007;389:1225–32. https://doi.org/10.1007/s00216-007-1517-3.
Nakamura H, Suzuki M. New concept for a toxicity assay based on multiple indexes from the wave shape of damped metabolic oscillation induced in living yeast cells – Part II: Application to analytical toxicology. Anal Bioanal Chem. 2007;389:1233–41. https://doi.org/10.1007/s00216-007-1513-7.
Ponomareva ON, Arlyapov VA, Alferov VA, Reshetilov AN. Microbial biosensors for detection of biological oxygen demand (a Review). Appl Biochem Microbiol. 2011;47:1–11. https://doi.org/10.1134/S0003683811010108.
Reshetilov AN, Arlyapov V, Alferov V, Reshetilova T. BOD biosensors: application of novel technologies and prospects for the development. In: Rinken T, editor. State of the art in biosensors – environmental and medical applications. Croatia: InTech; 2013. https://doi.org/10.5772/52385.
Jouanneau S, Recoules L, Durand MJ, Boukabache A, Picot V, Primault Y, et al. Methods for assessing biochemical oxygen demand (BOD): a review. Water Res. 2014;49:62–82. https://doi.org/10.1016/j.watres.2013.10.066.
Hassan SHA, Van Ginkel SW, Hussein MAM, Abskharon R, Oh SE. Toxicity assessment using different bioassays and microbial biosensors. Environ Int. 2016;92-93:106–18. https://doi.org/10.1016/j.envint.2016.03.003.
Nakanishi K, Ikebukuro K, Karube I. Determination of cyanide using a microbial sensor. Appl Biochem Biotechnol. 1996;60:97–106. https://doi.org/10.1007/BF02788064.
Ikebukuro K, Nakamura H, Karube I. Cyanides. In: Nollet LML, editor. Handbook of water analysis. New York: Marcel Dekker; 2000. p. 367–85.
Chang JC, Taylor PB, Leach FR. Use of the Microtox assay system for environmental samples. Bull Environ Contam Toxicol. 1981;26:150–6.
Keane A, Phoenix P, Ghoshal S, Lau PCK. Exposing culprit organic pollutants: a review. J Microbiol Methods. 2002;49:103–19. https://doi.org/10.1016/S0167-7012(01)00382-7.
Bulich AA, Isenberg DL. Use of the luminescent bacterial system for the rapid assessment of aquatic toxicity. ISA Trans. 1981;20:29–33.
Hyun CK, Tamiya E, Takeuchi T, Karube I. A novel BOD sensor based on bacterial luminescence. Biotechnol Bioeng. 1993;41:1107–11. http://europepmc.org/abstract/med/7251338.
Van Dyk TK, Majarian WR, Konstantinov KB, Young RM, Dhurjati PS, LaRossa RA. Rapid and sensitive pollutant detection by induction of heat shock gene-bioluminescence gene fusions. Appl Environ Microbiol. 1994;60:1414–20. http://aem.asm.org/content/60/5/1414.short
Belkin S, Smulski DR, Dadon S, Vollmer AC, Van Dyk TK, LaRossa RA. A panel of stress-responsive luminous bacteria for the detection of selected classes of toxicants. Water Res. 1997;31:3009–16. https://doi.org/10.1016/S0043-1354(97)00169-3.
Virta M, Lampinen J, Karp M. A luminescence-based mercury biosensor. Anal Chem. 1995;67:667–9. https://doi.org/10.1021/ac00099a027.
Walmsley RM, Billinton N, Heyer WD. Green fluorescent protein as a reporter for the DNA damage-induced gene RAD54 in Saccharomyces cerevisiae. Yeast. 1997;13:1535–45. https://doi.org/10.1002/(SICI)1097-0061(199712)13:16<;1535::AID-YEA221>;3.0.CO;2-2.
Roda A. Chemiluminescence and Bioluminescence: Past, present, and future. London: RSC Publishing; 2010.
Woutersen M, Belkin S, Brouwer B, Wezel AP, Minne B, Heringa MB. Are luminescent bacteria suitable for online detection and monitoring of toxic compounds in drinking water and its sources? Anal Bioanal Chem. 2011;400:915–29. https://doi.org/10.1007/s00216-010-4372-6.
Roda A, Guardigli M. Analytical chemiluminescence and bioluminescence: latest achievements and new horizons. Anal Bioanal Chem. 2012;402:69–76. https://doi.org/10.1007/s00216-011-5455-8.
Xiao Y, De Araujo C, Sze CC, Stuckey DC. Toxicity measurement in biological wastewater treatment processes: a review. J Hazard Mater. 2015;286:15–29. https://doi.org/10.1016/j.jhazmat.2014.12.033.
Roda A, Mirasoli M, Michelini E, Fusco MD, Zangheri M, Cevenini L, et al. Progress in chemical luminescence-based biosensors: a critical review. Biosens Bioelectron. 2016;76:164–79. https://doi.org/10.1016/j.bios.2015.06.017.
Thouand G, Marks RS. Bioluminescent microbial biosensors: design, construction, and implementation. Singapore: Pan Stanford Publishing Pte. Ltd.; 2016.
Ahn JM, Kim JH, Kim JH, Gu MB. Randomly distributed arrays of optically coded functional microbeads for toxicity screening and monitoring. Lab Chip. 2010;10:2695–701. https://doi.org/10.1039/C004942E.
Ahn JM, Gu MB. Geno-Tox: cell array biochip for genotoxicity monitoring and classification. Appl Biochem Biotechnol. 2012;168:752–60. https://doi.org/10.1007/s12010-012-9815-4.
Jung I, Seo HB, Lee J, Kim BC, Gu MB. A dip-stick type biosensor using bioluminescent bacteria encapsulated in color-coded alginate microbeads for detection of water toxicity. Analyst. 2014;139:4696–701. https://doi.org/10.1039/C4AN00308J.
Eltzov E, Cohen A, Marks RS. Bioluminescent liquid light guide pad biosensor for indoor air toxicity monitoring. Anal Chem. 2015;87:3655–61. https://doi.org/10.1021/ac5038208.
Eltzov E, Yehuda A, Marks RS. Creation of a new portable biosensor for water toxicity determination. Sensors Actuators B Chem. 2015;221:1044–54. https://doi.org/10.1016/j.snb.2015.06.153.
Axelrod T, Eltzov E, Marks RS. Bioluminescent bioreporter pad biosensor for monitoring water toxicity. Talanta. 2016;149:290–7. https://doi.org/10.1016/j.talanta.2015.11.067.
Mazzai A, Eltzov E, Manzano M, Marks RS. Probing putative carcinogenic potential of processed and unprocessed meat using bioluminescent bacterial bioreporters. Sensors Actuators B Chem. 2017;239:113–9. https://doi.org/10.1016/j.snb.2016.07.180.
Roda A, Cevenini L, Michelini E, Branchini BR. A portable bioluminescence engineered cell-based biosensor for on-site applications. Biosens Bioelectron. 2011;26:3647–53. https://doi.org/10.1016/j.bios.2011.02.022.
Cevenini L, Calabretta MM, Tarantino G, Michelini E, Roda A. Smartphone-interfaced 3D printed toxicity biosensor integrating bioluminescent “sentinel cells”. Sensors Actuators B Chem. 2016;225:249–57. https://doi.org/10.1016/j.snb.2015.11.017.
Cevenini L, Calabretta MM, Lopreside A, Tarantino G, Ferri M, Roda A, et al. Exploiting NanoLuc luciferase for smartphone-based bioluminescence cell biosensor for (anti)-inflammatory activity and toxicity. Anal Bioanal Chem. 2016;408:8859–68. https://doi.org/10.1007/s00216-016-0062-3.
Roda A, Cevenini L, Borg S, Michelini E, Calabretta MM, Schüler D. Bioengineered bioluminescent magnetotactic bacteria as a powerful tool for chip-based whole-cell biosensors. Lab Chip. 2013;13:4881–9. https://doi.org/10.1039/C3LC50868D.
Charrier T, Durand MJ, Jouanneau S, Dion M, Pernetti M, Poncelet D, et al. A multi-channel bioluminescent bacterial biosensor for the on-line detection of metals and toxicity. Part I: Design and optimization of bioluminescent bacterial strains. Anal Bioanal Chem. 2011;400:1051–60. https://doi.org/10.1007/s00216-010-4353-9.
Charrier T, Chapeau C, Bendria L, Picart P, Daniel P, Thouand G. A multi-channel bioluminescent bacterial biosensor for the on-line detection of metals and toxicity. Part II: Technical development and proof of concept of the biosensor. Anal Bioanal Chem. 2011;400:1061–70. https://doi.org/10.1007/s00216-010-4354-8.
Horry H, Charrier T, Durand MJ, Vrignaud B, Picart P, Daniel P, et al. Technological conception of an optical biosensor with a disposable card for use. Sensors Actuators B Chem. 2007;122:527–34. https://doi.org/10.1016/j.snb.2006.06.033.
Jouanneau S, Durand MJ, Courcoux P, Blusseau T, Thouand G. Improvement of the identification of four heavy metals in environmental samples by using predictive decision tree models coupled with a set of five bioluminescent bacteria. Environ Sci Technol. 2011;45:2925–31. dx.doi.org. https://doi.org/10.1021/es1031757.
Jouanneau S, Durand MJ, Thouand G. Online detection of metals in environmental samples: comparing two concepts of bioluminescent bacterial biosensors. Environ Sci Technol. 2012;46:11979–87. https://doi.org/10.1021/es3024918.
Affi M, Solliec C, Legentilhomme P, Comiti J, Legrand J, Jouanneau S, et al. Numerical modeling of the dynamic response of a bioluminescent bacterial biosensor. Anal Bioanal Chem. 2016;408:8761–70. https://doi.org/10.1007/s00216-016-9490-3.
Jouanneau S, Durand-Thouand MJ, Thouand G. Design of a toxicity biosensor based on Aliivibrio fischeri entrapped in a disposable card. Environ Sci Pollut Res. 2016;23:4340–5. https://doi.org/10.1007/s11356-015-4942-4.
Prévéral S, Brutesco C, Descamps ECT, Escoffier C, Pignol D, Ginet N, et al. A bioluminescent arsenite biosensor designed for inline water analyzer. Environ Sci Pollut Res. 2017;24:25–32. https://doi.org/10.1007/s11356-015-6000-7.
Tseng HW, Tsai YJ, Yen JH, Chen PH, Yeh YC. A fluorescence-based microbial sensor for the selective detection of gold. Chem Commun. 2014;50:1735–7. https://doi.org/10.1039/C3CC48028C.
Jha RK, Kern TL, Kim Y, Tesar C, Jedrzejczak R, Joachimiak A, et al. A microbial sensor for organophosphate hydrolysis exploiting an engineered specificity switch in a transcription factor. Nucleic Acids Res. 2016;44:8490–500. https://doi.org/10.1093/nar/gkw687.
Lee WI, Shrivastava S, Duy LT, Kim BY, Son YM, Lee NE. A smartphone imaging-based label-free and dual-wavelength fluorescent biosensor with high sensitivity and accuracy. Biosens Bioelectron. 2017;94:643–50. https://doi.org/10.1016/j.bios.2017.03.061.
Nakamura H, Murakami Y, Yokoyama K, Tamiya E, Karube I, Suda M, et al. A compactly integrated flow cell with a chemiluminescent FIA system for determining lactate concentration in serum. Anal Chem. 2001;73:373–8. https://doi.org/10.1021/ac000855u.
Roggo C, Meer JR. Miniaturized and integrated whole cell living bacterial sensors in field applicable autonomous devices. Curr Opin Biotechnol. 2017;45:24–33. https://doi.org/10.1016/j.copbio.2016.11.023.
Kou S, Cheng D, Sun F, Hsing IM. Microfluidics and microbial engineering. Lab Chip. 2016;16:432–46. https://doi.org/10.1039/C5LC01039J.
Recoules L, Migaou A, Dollat X, Thouand G, Gue AM, Boukabache A. A MEMS approach to determine the biochemical oxygen demand (BOD) of wastewaters. J Micromech Microeng. 2017;27:075018. https://doi.org/10.1088/1361-6439/aa710e.
Choi S. Microscale microbial fuel cells: Advances and challenges. Biosens Bioelectron. 2015;69:8–25. https://doi.org/10.1016/j.bios.2015.02.021.
Dávila D, Esquivel JP, Sabaté N, Mas J. Silicon-based microfabricated microbial fuel cell toxicity sensor. Biosens Bioelectron. 2011;26:2426–30. https://doi.org/10.1016/j.bios.2010.10.025.
Zhao X, Dong T. A microfluidic device for continuous sensing of systemic acute toxicants in drinking water. Int J Environ Res Public Health. 2013;10:6748–63. https://doi.org/10.3390/ijerph10126748.
Yeom SH, Kang BH, Kim KJ, Kang SW. Nanostructures in biosensor– a review. Front Biosci. 2011;16:997–1023. https://doi.org/10.2741/3731.
Lim JW, Ha D, Lee J, Lee SK, Kim T. Review of micro/nanotechnologies for microbial biosensors. Front Bioeng Biotechnol. 2015;3:61. https://doi.org/10.3389/fbioe.2015.00061.
Melamed S, Elad T, Belkin S. Microbial sensor cell arrays. Curr Opin Biotechnol. 2012;23:2–8. https://doi.org/10.1016/j.copbio.2011.11.024.
Wegener J. Cell-based microarrays for in vitro toxicology. Annu Rev Anal Chem. 2015;8:335–58. https://doi.org/10.1146/annurev-anchem-071213-020051.
Kaimori S, Kitamura T, Ichino M, Hosoya T, Kurusu F, Ishikawa T, et al. Structural development of a minimally invasive sensor chip for blood glucose monitoring. Anal Chim Acta. 2006;573-574:104–9. https://doi.org/10.1016/j.aca.2006.03.005.
Kitamura T, Kaimori S, Harada A, Ishikawa T, Fujimura T, Nakamura H, et al. Development of blood glucose monitoring sensor strip that uses small blood sample. SEI Technical Rev. 2006;63:19–21. http://www.sei.co.jp/technology/tr/pdf/sei10474.pdf.
Nakamura H, Tohyama K, Tanaka M, Shinohara S, Tokunaga Y, Kurusu F, et al. Development of a package-free transparent disposable biosensor chip for simultaneous measurements of blood constituents and investigation of its storage stability. Biosens Bioelectron. 2007;23:621–6. https://doi.org/10.1016/j.bios.2007.07.006.
Gotoh M, Hirose H, Ishikawa T, Nakamura H, Yokoyama K. Establishment of ferricyanide chronoamperometric total antioxidant capacity assay employing a carbon screen-printed disposable microchip-fundamental study using vegetable extraction. Sens Material. 2015;27:825–38. https://doi.org/10.18494/SAM.2015.1119.
Nakamura H. Google scholar cite. https://scholar.google.com/citations?user=Cesgwz8AAAAJ&hl=en
Alonso-Lomillo MA, Domínguez-Renedo O, Arcos-Martínez MJ. Screen-printed biosensors in microbiology; a review. Talanta. 2010;82:1629–36. https://doi.org/10.1016/j.talanta.2010.08.033.
Cui Y. Electronic materials, devices, and signals in electrochemical sensors. IEEE Trans Electron Device. 2017;64:1–11. https://doi.org/10.1109/TED.2017.2691045.
Erable B, Duţeanu NM, Ghangrekar MM, Dumas C, Scott K. Application of electro-active biofilms. Biofouling. 2010;26:57–71. https://doi.org/10.1080/08927010903161281.
Patil S, Harnisch F, Schröder U. Toxicity response of electroactive microbial biofilms—a decisive feature for potential biosensor and power source applications. Chem Phys Chem. 2010;11:2834–7. https://doi.org/10.1002/cphc.201000218.
Yoetz-Kopelman T, Dror Y, Shacham-Diamand Y, Freeman A. “Cells-on-Beads”: a novel immobilization approach for the construction of whole-cell amperometric biosensors. Sensors Actuators B Chem. 2016;232:758–64. https://doi.org/10.1016/j.snb.2016.03.132.
Yoetz-Kopelman T, Pandey R, Freeman A, Shacham-Diamand Y. Modeling of suspended versus immobilized whole-cell amperometric biosensors. Sensors Actuators B Chem. 2017;238:1248–57. https://doi.org/10.1016/j.snb.2016.09.062.
Székács A, Trummer N, Adányi N, Váradi M, Szendrő I. Development of a non-labeled immunosensor for the herbicide trifluralin via optical waveguide light mode spectroscopic detection. Anal Chim Acta. 2003;487:31–42. https://doi.org/10.1016/S0003-2670(03)00302-7.
Nakamura H, Mogi Y, Akimoto T, Naemura K, Kato T, Yano K, et al. An enzyme-chromogenic surface plasmon resonance biosensor probe for hydrogen peroxide determination using a modified Trinder's reagent. Biosens Bioelectron. 2008;24:455–60. https://doi.org/10.1016/j.bios.2008.04.022.
Adányi N, Bori Z, Szendrő I, Erdélyi K, Wang X, Schröder HC, et al. Biosilica-based immobilization strategy for label-free OWLS sensors. Sensors Actuators B Chem. 2013;177:1–7. https://doi.org/10.1016/j.snb.2012.10.116.
Adányi N, Bori Z, Szendrő I, Erdélyi K, Wang X, Schröder HC, et al. Bacterial sensors based on biosilica immobilization for label-free OWLS detection. New Biotechnol. 2013;30:493–9. https://doi.org/10.1016/j.nbt.2013.01.006.
Schloßmacher U, Wiens M, Schröder HC, Wang X, Jochum KP, Müller WEG. Silintaphin-1 interaction with silicatein during structure-guiding bio-silica formation. FEBS J. 2011;278:1145–55. https://doi.org/10.1111/j.1742-4658.2011.08040.x.
Kashem MA, Suzuki M, Kimoto K, Iribe Y. An optical biochemical oxygen demand biosensor chip for environmental monitoring. Sensors Actuators B Chem. 2015;221:1594–600. https://doi.org/10.1016/j.snb.2015.07.119.
Ivandini TA, Saepudin E, Wardah H, Dewangga N, Einaga Y. Development of a biochemical oxygen demand sensor using gold-modified boron doped diamond electrodes. Anal Chem. 2012;84:9825–32. https://doi.org/10.1021/ac302090y.
Yamashita T, Ookawa N, Ishida M, Kanamori H, Sasaki H, Katayose Y, et al. A novel open-type biosensor for the in-situ monitoring of biochemical oxygen demand in an aerobic environment. Sci Rep. 2016;6:38552. https://doi.org/10.1038/srep38552.
Nakano R, Kuroki Y, Shimojo M, Kawakami M. Evaluation of biochemical oxygen demand for wastewater based on. Res Bull Fukuoka Inst Technol. 2016;49:1–7. http://hdl.handle.net/11478/545
Šefčovičová J, Filip J, Mastihuba V, Gemeiner P, Tkac J. Analysis of ethanol in fermentation samples by a robust nanocomposite-based microbial biosensor. Biotechnol Lett. 2012;34:1033–9. https://doi.org/10.1007/s10529-012-0875-x.
Šefčovičová J, Filip J, Tkac J. Interfacing of microbial cells with nanoparticles: Simple and cost-effective preparation of a highly sensitive microbial ethanol biosensor. Chem Pap. 2015;69:176–82. https://doi.org/10.1515/chempap-2015-0012.
Aslan S, Anik Ü. Microbial glucose biosensors based on glassy carbon paste electrodes modified with Gluconobacter oxydans and graphene oxide or graphene-platinum hybrid nanoparticles. Microchim Acta. 2016;183:73–81. https://doi.org/10.1007/s00604-015-1590-9.
Arlyapov VA, Kamanin S, Ponamoreva ON, Reshetilov AN. Biosensor analyzer for BOD index express control on the basis of the yeast microorganisms Candida maltosa, Candida blankii, and Debaryomyces hansenii. Enzym Microb Technol. 2012;50:215–20. https://doi.org/10.1016/j.enzmictec.2012.01.002.
Arlyapov VA, Yudina NY, Asulyan LD, Alferov SV, Alferov VA, Reshetilov AN. BOD biosensor based on the yeast Debaryomyces hansenii immobilized in poly (vinyl alcohol) modified by N-vinylpyrrolidone. Enzym Microb Technol. 2013;53:257–62. https://doi.org/10.1016/j.enzmictec.2013.05.004.
Ponamoreva ON, Kamanina OA, Alferov VA, Machulin AV, Rogova TV, Arlyapov VA, et al. Yeast-based self-organized hybrid biosilica sol–gels for the design of biosensors. Biosens Bioelectron. 2015;67:321–6. https://doi.org/10.1016/j.bios.2014.08.045.
Raudkivi K, Tutt M, Talpsep E, Kikas T. Pseudomonas putida P67. 2 and Pseudomonas flourescens P75 based microbial sensors for biochemical oxygen demand (BOD) measurements in phenolic wastewaters of oil shale industry. Oil Shale. 2008;25:376–86.
Raud M, Linde E, Kibena E. S Velling S, Tenn T, Talpsep E, Kikas T. Semi-specific biosensors for measuring BOD in dairy wastewater. J Chem Technol Biotechnol. 2010;85:957–61. https://doi.org/10.1002/jctb.2385.
Raud M, Tenno T, Jõgi E, Kikas T. Comparative study of semi-specific Aeromonas hydrophila and universal Pseudomonas fluorescens biosensors for BOD measurements in meat industry wastewaters. Enzym Microb Technol. 2012;50:221–6. https://doi.org/10.1016/j.enzmictec.2012.01.003.
Raud M, Tutt M, Jõgi E, Kikas T. BOD biosensors for pulp and paper industry wastewater analysis. Environ Sci Pol. 2012;19:3039–45. https://doi.org/10.1007/s11356-012-0817-0.
Kibena E, Raud M, Jõgi E, Kikas T. Semi-specific Microbacterium phyllosphaerae-based microbial sensor for biochemical oxygen demand measurements in dairy wastewater. Environ Sci Pol. 2013;20:2492–8. https://doi.org/10.1007/s11356-012-1166-8.
Raud M, Kikas T. Bioelectronic tongue and multivariate analysis: a next step in BOD measurements. Water Res. 2013;47:2555–62. https://doi.org/10.1016/j.watres.2013.02.026.
Pitman K, Raud M, Kikas T. Biochemical oxygen demand sensor arrays. Agron Res. 2015;13:382–95.
Li Y, Sun J, Wang J, Bian C, Tong J, Li Y, et al. A single-layer structured microbial sensor for fast detection of biochemical oxygen demand. Biochem Eng J. 2016;112:219–25. https://doi.org/10.1016/j.bej.2016.04.021.
Li Y, Sun J, Wang J, Bian C, Tong J, Li Y, et al. A microbial electrode based on the co-electrodeposition of carboxyl graphene and Au nanoparticles for BOD rapid detection. Biochem Eng J. 2017;123:86–94. https://doi.org/10.1016/j.bej.2017.03.015.
Hsieh MC, Chung YC. Measurement of biochemical oxygen demand from different wastewater samples using a mediatorless microbial fuel cell biosensor. Environ Technol. 2014;35:2204–11. https://doi.org/10.1080/09593330.2014.898700.
Hsieh MC, Cheng CY, Liu MH, Chung YC. Effects of operating parameters on measurements of biochemical oxygen demand using a mediatorless microbial fuel cell biosensor. Sensors. 2016;16:35. https://doi.org/10.3390/s16010035.
Webber JB, Noonan M, Pasco NF, Hay JM. Appraising bacterial strains for rapid BOD sensing—an empirical test to identify bacterial strains capable of reliably predicting real effluent BODs. Appl Microbiol Biotechnol. 2011;89:179–88. https://doi.org/10.1007/s00253-010-2889-4.
Czolkos I, Dock E, Tønning E, Christensen J, Winther-Nielsene M, Carlsson C, et al. Prediction of wastewater quality using amperometric bioelectronic tongues. Biosens Bioelectron. 2016;75:375–82. https://doi.org/10.1016/j.bios.2015.08.055.
Cetó X, Voelcker NH, Prieto-Simón B. Bioelectronic tongues: new trends and applications in water and food analysis. Biosens Bioelectron. 2016;79:608–26. https://doi.org/10.1016/j.bios.2015.12.075.
Valle M. Bioelectronic tongues employing electrochemical biosensors. TRE Bioelectroanal. 2016;6:143–202. https://doi.org/10.1007/11663_2016_2.
Zhang Y, Angelidaki I. Submersible microbial fuel cell sensor for monitoring microbial activity and BOD in groundwater: focusing on impact of anodic biofilm on sensor applicability. Biotechnol Bioeng. 2011;108:2339–47. https://doi.org/10.1002/bit.23204.
Peixoto L, Min B, Martins G, Brito AG, Kroff P, Parpot P, et al. In situ microbial fuel cell-based biosensor for organic carbon. Bioelectrochemistry. 2011;81:99–103. https://doi.org/10.1016/j.bioelechem.2011.02.002.
Yang GX, Sun YM, Kong XY, Zhen F, Li Y, Li LH, et al. Factors affecting the performance of a single-chamber microbial fuel cell-type biological oxygen demand sensor. Water Sci Technol. 2013;68:1914–9. https://doi.org/10.2166/wst.2013.415.
Ayyaru S, Dharmalingam S. Enhanced response of microbial fuel cell using sulfonated poly ether ether ketone membrane as a biochemical oxygen demand sensor. Anal Chim Acta. 2014;818:15–22. https://doi.org/10.1016/j.aca.2014.01.059.
Ayyaru S, Dharmalingam S. A study of influence on nanocomposite membrane of sulfonated TiO2 and sulfonated polystyrene-ethylene-butylene-polystyrene for microbial fuel cell application. Energy. 2015;88:202–8. https://doi.org/10.1016/j.energy.2015.05.015.
Logroño W, Guambo A, Pérez M, Kadier A, Recalde C. A terrestrial single chamber microbial fuel cell-based biosensor for biochemical oxygen demand of synthetic rice washed wastewater. Sensors. 2016;16:101. https://doi.org/10.3390/s16010101.
Kharkwal S, Tan YC, Lu M, Ng HY. Development and long-term stability of a novel microbial fuel cell BOD sensor with MnO2 catalyst. Int J Mol Sci. 2017;18:276. https://doi.org/10.3390/ijms18020276.
Anam M, Yousaf S, Sharafat I, Zafar Z, Ayaz K, Ali N. Comparing natural and artificially designed bacterial consortia as biosensing elements for rapid non-specific detection of organic pollutant through microbial fuel cell. Int J Electrochem Sci. 2017;12:2836–51. https://doi.org/10.20964/2017.04.49.
Nalewajko C, Lean DRS. Growth and excretion in planktonic algae and bacteria. J Phycol. 1972;8:361–6. https://doi.org/10.1111/j.1529-8817.1972.tb04049.x.
Munster U, Chrost RJ. Origin, composition, and microbial utilization of dissolved organic matter. In: Overbeck J, Chrost RJ, editors. Aquatic microbial ecology. New York: Springer; 1990. p. 8–46. https://doi.org/10.1007/978-1-4612-3382-4_2.
Shimotori K, Omori Y, Hama T. Bacterial production of marine humic-like fluorescent dissolved organic matter and its biogeochemical importance. Aquat Microb Ecol. 2010;58:55–66. https://doi.org/10.3354/ame01350.
Tranvik LJ. Microbial transformation of labile dissolved organic matter into humic-like matter in seawater. FEMS Microbiol Ecol. 1993;12:177–83. https://doi.org/10.1111/j.1574-6941.1993.tb00030.x.
Kobayashi S, Nakada S, Nakajima M, Yamamoto K, Akiyama S, Fuchi M, et al. Visualization of the distribution of dissolved organic matter in Osaka Bay using a satellite ocean color sensor (COMS/GOCI). J Water Environ Technol. 2017;15:55–64. https://doi.org/10.2965/jwet.16-055.
Chen HY, Guan YX, Yao SJ. A novel two-species whole-cell immobilization system composed of marine-derived fungi and its application in wastewater treatment. J Chem Technol. 2014;89:1733–40. https://doi.org/10.1002/jctb.4253.
Chen HY, Wang M, Shen Y, Yao SJ. Optimization of two-species whole-cell immobilization system constructed with marine-derived fungi and its biological degradation ability. Chin J Chem Eng. 2014;22:187–92. https://doi.org/10.1016/S1004-9541(14)60024-0.
Górski Ł, Trzebuniak KF, Elżbieta M. Low bod determination methods: the state-of-the-art. Chem Process Eng. 2012;33:629–37. https://doi.org/10.2478/v10176-012-0053-7.
Balootaki PA, Hassanshahian M. Microbial biosensor for marine environments. Bull Environ Pharmacol Life Sci. 2014;3:1–13.
Cheng L, QueK SB, Cord-Ruwisch R. Hexacyanoferrate-adapted biofilm enables the development of a microbial fuel cell biosensor to detect trace levels of assimilatable organic carbon (AOC) in oxygenated seawater. Biotechnol Bioeng. 2014;111:2412–20. https://doi.org/10.1002/bit.25315.
Quek SB, Cheng L, Cord-Ruwisch R. Microbial fuel cell biosensor for rapid assessment of assimilable organic carbon under marine conditions. Water Res. 2015;77:64–71. https://doi.org/10.1016/j.watres.2015.03.012.
Quek SB, Cheng L, Cord-Ruwisch R. Detection of low concentration of assimilatable organic carbon in seawater prior to reverse osmosis membrane using microbial electrolysis cell biosensor. Desalinat Water Treat. 2015;55:2885–90. https://doi.org/10.1080/19443994.2014.940224.
Quek SB, Cheng L, Cord-Ruwisch R. In-line deoxygenation for organic carbon detections in seawater using a marine microbial fuel cell-biosensor. Bioresour Technol. 2015;182:34–40. https://doi.org/10.1016/j.biortech.2015.01.078.
Ebrahimi A, Yousefi Kebria D, Darzi GN. Improving bioelectricity generation and COD removal of sewage sludge in microbial desalination cell. Environ Technol 2017:1–10. https://doi.org/10.1080/09593330.2017.1323958
Chee GJ. Development and characterization of microbial biosensors for evaluating low biochemical oxygen demand in rivers. Talanta. 2013;117:366–70. https://doi.org/10.1016/j.talanta.2013.09.031.
Liu C, Zhao H, Ma Z, An T, Liu C, Zhao L, et al. Novel environmental analytical system based on combined biodegradation and photoelectrocatalytic detection principles for rapid determination of organic pollutants in wastewaters. Environ Sci Technol. 2014;48:1762–8. https://doi.org/10.1021/es4031358.
Namour P, Jaffrezic-Renault N. Sensors for measuring biodegradable and total organic matter in water. Trends Anal Chem. 2010;29:848–57. https://doi.org/10.1016/j.trac.2010.04.013.
Hong SW, Kim HS, Chung TH. Alteration of sediment organic matter in sediment microbial fuel cells. Environ Pollut. 2010;158:185–91. https://doi.org/10.1016/j.envpol.2009.07.022.
Liu Z, Liu J, Zhang S, Xing XH, Su Z. Microbial fuel cell based biosensor for in situ monitoring of anaerobic digestion process. Bioresour Technol. 2011;102:10221–9. https://doi.org/10.1016/j.biortech.2011.08.053.
Liang B, Li L, Tang XJ, Lang Q, Wang H, Li F, et al. Microbial surface display of glucose dehydrogenase for amperometric glucose biosensor. Biosens Bioelectron. 2013;45:19–24. https://doi.org/10.1016/j.bios.2013.01.050.
Golitsch F, Bücking C, Gescher J. Proof of principle for an engineered microbial biosensor based on Shewanella oneidensis outer membrane protein complexes. Biosens Bioelectron. 2013;47:285–91. https://doi.org/10.1016/j.bios.2013.03.010.
Nomura Y, Ikebukuro K, Yokoyama K, Takeuchi T, Arikawa Y, Ohno S, et al. A novel microbial sensor for anionic surfactant determination. Anal Lett. 1994;27:3095–108. https://doi.org/10.1080/00032719408000313.
Nomura Y, Ikebukuro K, Yokoyama K, Takeuchi T, Arikawa Y, Ohno S, et al. Application of a linear alkylbenzene sulfonate biosensor to river water monitoring. Biosens Bioelectron. 1998;13:1047–53. https://doi.org/10.1016/S0956-5663(97)00077-8.
Zhang Q, Xia YF, Hong JM. Mechanism and toxicity research of benzalkonium chloride oxidation in aqueous solution by H2O2/Fe2+ process. Environ Sci Pollut Res. 2016;23:17822–30. https://doi.org/10.1007/s11356-016-6986-5.
Okada T, Karube I, Suzuki S. Microbial sensor system which uses Methylomonas sp. for the determination of methane. Appl Microbiol Biotechnol. 1981;12:102–6. https://doi.org/10.1007/BF01970042.
Karube I, Okada T, Suzuki S. A methane gas sensor based on oxidizing bacteria. Anal Chim Acta. 1982;135:61–7. https://doi.org/10.1016/S0003-2670(01)85265-X.
Damgaard LR, Revsbech NP. A microscale biosensor for methane containing methanotrophic bacteria and an internal oxygen reservoir. Anal Chem. 1997;69:2262–7. https://doi.org/10.1021/ac9611576.
Zarei M, Farahbakhsh A. A modified optical microbial biosensor for detection of methane using gold nanoparticle and methanotrophic bacteria. J Materials Sci Surface Eng. 2016;4:335–8.
Baronian KHR, Gurazada S. Electrochemical detection of wild type Saccharomyces cerevisiae responses to estrogens. Biosens Bioelectron. 2007;22:2493–9. https://doi.org/10.1016/j.bios.2006.09.031.
Plekhanova YV, Reshetilov AN, Manolov TV, Taranova LA. Biosensor monitoring of microbial treatment of wastewater from nonylphenol polyethoxylates under flow-through conditions. Appl Biochem Microbiol. 2011;47:846–51. https://doi.org/10.1134/S0003683811090043.
Bazin I, Seo HB, Suehs CM, Ramuz M, De Waad M, Gu MB. Profiling the biological effects of wastewater samples via bioluminescent bacterial biosensors combined with estrogenic assays. Environ Sci Pollut Res. 2017;24:33–41. https://doi.org/10.1007/s11356-016-6050-5.
Villalba MM, McKeegan KJ, Vaughan DH, Cardosi MF, Davis J. Bioelectroanalytical determination of phosphate: a review. J Mol Catal B Enzym. 2009;59:1–8. https://doi.org/10.1016/j.molcatb.2008.12.011.
Nakamura H. Phosphate ion determination in water for drinking using biosensors. Bunseki Kagaku. 2001;50:581–2. https://doi.org/10.2116/bunsekikagaku.50.581.
Ikebukuro K, Nishida R, Yamamoto H, Arikawa Y, Nakamura H, Suzuki M, et al. A novel biosensor system for the determination of phosphate. J Biotechnol. 1996;48:67–72. https://doi.org/10.1016/0956-5663(96)87655-X.
Ikebukuro K, Nakamura H, Karube I, Kubo I, Inagawa M, Sugawara T, et al. Phosphate sensing system using pyruvate oxidase and chemiluminescence detection. Biosens Bioelectron. 1996;11:959–65. https://doi.org/10.1016/0168-1656(96)01398-3.
Nakamura H, Ikebukuro K, McNiven S, Karube I, Yamamoto H, Hayashi K, et al. A chemiluminescent FIA biosensor for phosphate ion monitoring using pyruvate oxidase. Biosens Bioelectron. 1997;12:959–66. https://doi.org/10.1016/S0956-5663(97)00032-8.
Suzuki M, Kurata H, Inoue Y, Shin H, Kubo I, Nakamura H, et al. Reagentless phosphate ion sensor system for environmental monitoring. Electrochemistry. 1998;66:579–83. http://iss.ndl.go.jp/books/R100000002-I000000031819-00
Nakamura H, Hasegawa M, Nomura Y, Ikebukuro K, Arikawa Y, Karube I. Improvement of a CL-FIA system using maltose phosphorylase for the determination of phosphate-ion in freshwater. Anal Lett. 2003;36:1805–17. https://doi.org/10.1081/AL-120023615.
Goron TL, Raizada MN. Current and future transgenic whole-cell biosensors for plant macro- and micronutrients. Crit Rev Plant Sci. 2014;33:392–413. https://doi.org/10.1080/07352689.2014.885733.
Gillor O, Hadas O, Post AF, Belkin S. Phosphorus bioavailability monitoring by a bioluminescent cyanobacterial sensor strain. J Phycol. 2002;38:107–15. https://doi.org/10.1046/j.1529-8817.2002.01069.x.
Gillor O, Harush A, Hadas O, Post AF, Belkin S. A synechococcus PglnA::luxAB fusion for estimation of nitrogen bioavailability to freshwater cyanobacteria. Appl Environ Microbiol. 2003;69:1465–74. https://doi.org/10.1128/AEM.69.3.1465-1474.2003.
Meer JR, Belkin S. Where microbiology meets microengineering: design and applications of reporter bacteria. Nat Rev Microbiol. 2010;8:511–22. https://doi.org/10.1038/nrmicro2392.
Elad T, Belkin S. Reporter gene assays in ecotoxicology. In: Reifferscheid G, Buchinger S, editors. In vitro in vitro environmental toxicology – concepts, application, and assessment. Berlin: Springer; 2017. p. 135–57.
Gillor O, Hadas O, Post AF, Belkin S. Phosphorus and nitrogen in a monomythic freshwater lake: employing cyanobacterial bioreporters to gain new insights into nutrient bioavailability. Freshwater Boil. 2010;55:1182–90. https://doi.org/10.1111/j.1365-2427.2009.02342.x.
Faraghi N, Ebrahimi S. Nitrite as a candidate substrate in microbial fuel cells. Biotechnol Lett. 2012;34:1483–6. https://doi.org/10.1007/s10529-012-0939-y.
Rezvani F, Sarrafzadeh MH, Ebrahimi S, Oh HM. Nitrate removal from drinking water with a focus on biological methods: a review. Environ Sci Pollut Res 2017:1–18. https://doi.org/10.1007/s11356-017-9185-0
Raud M, Lember E, Jõgi E, Kikas T. Nitrosomonas sp. based biosensor for ammonium nitrogen measurement in wastewater. Biotechnol Bioprocess Eng. 2013;18:1016–21. https://doi.org/10.1007/s12257-013-0078-x.
Zuki SNSM, Tan LL, Azmi NS, Heng LY, Chong KF, Tajuddin SN. A whole cell bio-optode based on immobilized nitrite-degrading microorganism on the acrylic microspheres for visual quantitation of nitrite ion. Sensors Actuators B Chem. 2018;255:2844–52. https://doi.org/10.1016/j.snb.2017.09.102.
Qi P, Zhang D, Wan Y. Development of an amperometric microbial biosensor based on Thiobacillus thioparus cells for sulfide and its application to detection of sulfate-reducing bacteria. Electroanalysis. 2014;26:1824–30. https://doi.org/10.1002/elan.201400198.
Ebrahimi E, Yazdian F, Amoabediny G, Shariati MR, Janfada B, Saber M. A microbial biosensor for hydrogen sulfide monitoring based on potentiometry. Process Biochem. 2014;49:1393–401. https://doi.org/10.1016/j.procbio.2014.05.003.
Vosoughi A, Yazdian F, Amoabediny G, Hakim M. Investigating the effect of design parameters on the response time of a highly sensitive microbial hydrogen sulfide biosensor based on oxygen consumption. Biosens Bioelectron. 2015;70:106–14. https://doi.org/10.1016/j.bios.2015.03.025.
Gupta V, Saharan K, Kumar L, Gupta R, Sahai V, Mittal A. Spectrophotometric ferric ion biosensor from Pseudomonas fluorescens culture. Biotechnol Bioeng. 2008;100:284–96. https://doi.org/10.1002/bit.21754.
Tran PHN, Luong TTT, Nguyen TTT, Nguyen HQ, Duong HV, Kim BH, et al. Possibility of using a lithotrophic iron-oxidizing microbial fuel cell as a biosensor for detecting iron and manganese in water samples. Environ Sci Processes Impacts. 2015;17:1806–15. https://doi.org/10.1039/C5EM00099H.
Liang PS, San Park T, Yoon JY. Rapid and reagentless detection of microbial contamination within meat utilizing a smartphone-based biosensor. Sci Rep. 2014;4:5953. https://doi.org/10.1038/srep05953.
Thouand G, Durand MJ. Bacteria in ecotoxicology: recombinant luminescent bacteria. Encyclopedia of aquatic ecotoxicology. New York: Springer; 2013. p. 137–50. https://doi.org/10.1007/978-94-007-5704-2_14.
Durand MJ, Hua A, Jouanneau S, Cregut M, Thouand G. Detection of metal and organometallic compounds with bioluminescent bacterial bioassays. In: Thouand G, Marks R, editors. Bioluminescence: fundamentals and applications in biotechnology. New York: Springer; 2015. p. 77–99. https://doi.org/10.1007/10_2015_332.
Jouanneau S, Durand MJ, Lahmar A, Thouand G. Main Technological Advancements in bacterial bioluminescent biosensors over the last two decades. In: Thouand G, Marks R, editors. Bioluminescence: fundamentals and applications in biotechnology. New York: Springer; 2015. p. 101–16. https://doi.org/10.1007/10_2015_333.
Jouanneau S, Durand MJ, Assaf A, Bittel M, Thouand G. Bacterial bioreporter applications in ecotoxicology: concepts and practical approach. Microb Ecotoxicol. 2017:283–311. https://doi.org/10.1007/978-3-319-61795-4_12.
Mankiewicz-Boczek J, Karwaciak I, Ratajewski M, Gągała I, Jurczak T, Zalewski M, et al. Application of cellular biosensors for detection of atypical toxic bioactivity in microcystin-containing cyanobacterial extracts. Aquat Toxicol. 2015;168:1–10. https://doi.org/10.1016/j.aquatox.2015.09.004.
Hassan SHA, Van Ginkel SW, Oh SE. Effect of organics and alkalinity on the sulfur oxidizing bacteria (SOB) biosensor. Chemoso. 2013;90:9655–970. https://doi.org/10.1016/j.chemosphere.2012.06.040.
Shen YJ, Lefebvre O, Tan Z, Ng Y. Microbial fuel cell-based toxicity sensor for fast monitoring of acidic toxicity. Water Sci Technol. 2012;65:1223–8. https://doi.org/10.2166/wst.2012.957.
Liu B, Lei Y, Li B. A batch-mode cube microbial fuel cell based “shock” biosensor for wastewater quality monitoring. Biosens Bioelectron. 2014;62:308–14. https://doi.org/10.1016/j.bios.2014.06.051.
Xu Z, Liu B, Dong Q, Lei Y, Li Y, Ren J, et al. Flat microliter membrane-based microbial fuel cell as “on-line sticker sensor” for self-supported in situ monitoring of wastewater shocks. Bioresour Technol. 2015;197:244–51. https://doi.org/10.1016/j.biortech.2015.08.081.
Xu Z, Liu Y, Williams I, Li Y, Qian F, Zhang H, et al. Disposable self-support paper-based multi-anode microbial fuel cell (PMMFC) integrated with power management system (PMS) as the real time “shock” biosensor for wastewater. Biosens Bioelectron. 2016;85:232–9. https://doi.org/10.1016/j.bios.2016.05.018.
Stein NE, Hamelers HVM, Buisman CNJ. Stabilizing the baseline current of a microbial fuel cell-based biosensor through overpotential control under non-toxic conditions. Bioelectrochemistry. 2010;78:87–91. https://doi.org/10.1016/j.bioelechem.2009.09.009.
Stein NE, Keesman KJ, Hamelers HVM, Straten G. Kinetic models for detection of toxicity in a microbial fuel cell based biosensor. Biosens Bioelectron. 2011;26:3115–20. https://doi.org/10.1016/j.bios.2010.11.049.
Stein NE, Hamelers HVM, Buisman CNJ. Influence of membrane type, current and potential on the response to chemical toxicants of a microbial fuel cell based biosensor. Sensors Actuators B Chem. 2012;163:1–7. https://doi.org/10.1016/j.snb.2011.10.060.
Stein NE, Hamelers HVM, Buisman CNJ. The effect of different control mechanisms on the sensitivity and recovery time of a microbial fuel cell-based biosensor. Sensors Actuators B Chem. 2012;171/172:816–21. https://doi.org/10.1016/j.snb.2012.05.076.
Stein NE, Hamelers HVM, Straten G, Keesman KJ. On-line detection of toxic components using a microbial fuel cell-based biosensor. J Process Control. 2012;22:1755–61. https://doi.org/10.1016/j.jprocont.2012.07.009.
Kim M, Hyun MS, Gadd GM, Kim HJ. A novel biomonitoring system using microbial fuel cells. J Environ Monit. 2007;9:1323–8. https://doi.org/10.1039/B713114C.
Labro J, Craig T, Wood SA, Packer MA. Demonstration of the use of a photosynthetic microbial fuel cell as an environmental biosensor. Int J Nanotechnol. 2017;14:1741–51. https://doi.org/10.1504/IJNT.2017.082467.
Jiang Y, Liang P, Liu P, Yan X, Bian Y, Huang X. A cathode-shared microbial fuel cell sensor array for water alert system. Int J Hydrog Energy. 2017;42:4342–8. https://doi.org/10.1016/j.ijhydene.2016.12.050.
Jiang Y, Liang P, Liu P, Wang D, Miao B, Huang X. A novel microbial fuel cell sensor with biocathode sensing element. Biosens Bioelectron. 2017;94:344–50. https://doi.org/10.1016/j.bios.2017.02.052.
Yu D, Bai L, Zhai J, Wang Y, Dong S. Toxicity detection in water containing heavy metal ions with a self-powered microbial fuel cell-based biosensor. Talanta. 2017;168:210–6. https://doi.org/10.1016/j.talanta.2017.03.048.
Zhang Q, Ding J, Kou K, Qin W. A potentiometric flow biosensor based on ammonia-oxidizing bacteria for the detection of toxicity in water. Sensors. 2013;13:6936–45. https://doi.org/10.3390/s130606936.
Bittel M, Cordella CBY, Assaf A, Jouanneau S, Durand MJ, Thouand G. Potential of Raman spectroscopy to monitor arsenic toxicity on bacteria: Insights toward multiparametric bioassays. Environ Sci Technol. 2015;49:12324–32. https://doi.org/10.1021/acs.est.5b03013.
Biswas P, Karn AK, Balasubramanian P, Kale PG. Biosensor for detection of dissolved chromium in potable water: a review. Biosens Bioelectron. 2017;94:589–604. https://doi.org/10.1016/j.bios.2017.03.043.
Merulla D, Buffi N, Beggah S, Truffer F, Geiser M, Renaud P, et al. Bioreporters and biosensors for arsenic detection. Biotechnological solutions for a world-wide pollution problem. Curr Opin Biotechnol. 2013;24:534–41. https://doi.org/10.1016/j.copbio.2012.09.002.
Chen J, Rosen BP. Biosensors for inorganic and organic arsenicals. Biosensors. 2014;4:494–512. https://doi.org/10.3390/bios4040494.
Kaur H, Kumar R, Babu JN, Mittal S. Advances in arsenic biosensor development – a comprehensive review. Biosens Bioelectron. 2015;63:533–45. https://doi.org/10.1016/j.bios.2014.08.003.
Shen Y, Wang M, Chang IS, Ng HY. Effect of shear rate on the response of microbial fuel cell toxicity sensor to Cu (II). Bioresour Technol. 2013;136:707–10. https://doi.org/10.1016/j.biortech.2013.02.069.
Chiou CH, Chien LJ, Chou TC, Lin JL, Tseng JT. Rapid whole-cell sensing chip for low-level arsenite detection. Biosens Bioelectron. 2011;26:2484–8. https://doi.org/10.1016/j.bios.2010.10.037.
Cortés-Salazar F, Beggah S, Meer JR, Girault HH. Electrochemical As(III) whole-cell based biochip sensor. Biosens Bioelectron. 2013;47:237–42. https://doi.org/10.1016/j.bios.2013.03.011.
Wang X, Liu M, Wang X, Wu Z, Yang L, Xia S. P-benzoquinone-mediated amperometric biosensor developed with Psychrobacter sp. for toxicity testing of heavy metals. Biosens Bioelectron. 2013;41:557–62. https://doi.org/10.1016/j.bios.2012.09.020.
Prabhakaran DC, Riotte J, Sivry Y, Subramanian S. Electroanalytical detection of Cr (VI) and Cr (III) ions using a novel microbial sensor. Electroanalysis. 2017;29:1222–31. https://doi.org/10.1002/elan.201600458.
Li L, Liang J, Hong W, Zhao Y, Sun S, Yang X, et al. Evolved bacterial biosensor for arsenite detection in environmental water. Environ Sci Technol. 2015;49:6149–55. https://doi.org/10.1021/acs.est.5b00832.
Asif S, Chaudhari A, Gireesh-Babu P, Chaudhuri PR, Sen R. Immobilization of fluorescent whole cell biosensors for the improved detection of heavy metal pollutants present in aquatic environment. Materials Today Proc. 2016;3:3492–7. https://doi.org/10.1016/j.matpr.2016.10.032.
Kim M, Lim JW, Kim HJ, Lee SK, Lee SJ, Kim T. Chemostat-like microfluidic platform for highly sensitive detection of heavy metal ions using microbial biosensors. Biosens Bioelectron. 2015;65:257–64. https://doi.org/10.1016/j.bios.2014.10.028.
Kim HJ, Lim JW, Jeong H, Lee SJ, Lee DW, Kim T, et al. Development of a highly specific and sensitive cadmium and lead microbial biosensor using synthetic CadC-T7 genetic circuitry. Biosens Bioelectron. 2016;79:701–8. https://doi.org/10.1016/j.bios.2015.12.101.
Kumar S, Verma N, Singh AK. Development of cadmium specific recombinant biosensor and its application in milk samples. Sensors Actuators B Chem. 2017;240:248–54. https://doi.org/10.1016/j.snb.2016.08.160.
Bereza-Malcolm L, Aracic S, Kannan R, Mann G, Franks AE. Functional characterization of Gram-negative bacteria from different genera as multiplex cadmium biosensors. Biosens Bioelecron. 2017;94:380–7. https://doi.org/10.1016/j.bios.2017.03.029.
Gammoudi I, Raimbault V, Tarbague H, Moroté F, Grauby-Heywang C, Othmane A, et al. Enhanced bio-inspired microsensor based on microfluidic/bacteria/love wave hybrid structure for continuous control of heavy metals toxicity in liquid medium. Sensors Actuators B Chem. 2014;198:278–84. https://doi.org/10.1016/j.snb.2014.01.104.
Gammoudi I, Tarbague H, Othmane A, Moynet D, Rebière D, Kalfat R, et al. Love-wave bacteria-based sensor for the detection of heavy metal toxicity in liquid medium. Biosens Bioelectron. 2010;26:1723–6. https://doi.org/10.1016/j.bios.2010.07.118.
Biran A, Yoav HB, Yagur-Kroll S, Pedahzur R, Buchinger S, Shacham-Diamand Y, et al. Microbial genotoxicity bioreporters based on sulA activation. Anal Bioanal Chem. 2011;400:3013–24. https://doi.org/10.1007/s00216-011-5007-2.
Hnaien M, Bourigua S, Bessueille F, Bausells J, Errachid A, Lagarde F, et al. Impedimetric microbial biosensor based on single wall carbon nanotube modified microelectrodes for trichloroethylene detection. Electrochim Acta. 2011;56:10353–8. https://doi.org/10.1016/j.electacta.2011.04.041.
Mulchandani A. Rajesh. Microbial biosensors for organophosphate pesticides. Appl Biochem Biotechnol. 2011;165:687–99. https://doi.org/10.1007/s12010-011-9288-x.
Verma N, Bhardwaj A. Biosensor technology for pesticides – a review. Appl Biochem Biotechnol. 2015;175:3093–119. https://doi.org/10.1007/s12010-015-1489-2.
Tang X, Zhang T, Liang B, Han D, Zeng L, Zheng C, et al. Sensitive electrochemical microbial biosensor for p-nitrophenyl organophosphates based on electrode modified with cell surface-displayed organophosphorus hydrolase and ordered mesopore carbons. Biosens Bioelectron. 2014;60:137–42. https://doi.org/10.1016/j.bios.2014.04.001.
Karim F, Fakhruddin ANM. Recent advances in the development of biosensor for phenol: a review. Rev Environ Sci Biotechnol. 2012;11:261–74. https://doi.org/10.1007/s11157-012-9268-9.
Kumar J, D'Souza SF. Microbial biosensor for detection of methyl parathion using screen printed carbon electrode and cyclic voltammetry. Biosens Bioelectron. 2011;26:4289–93. https://doi.org/10.1016/j.bios.2011.04.027.
Chen Z, Niu Y, Zhao S, Khan A, Ling Z, Chen Y, et al. A novel biosensor for p-nitrophenol based on an aerobic anode microbial fuel cell. Biosens Bioelectron. 2016;85:860–8. https://doi.org/10.1016/j.bios.2016.06.007.
Di Gennaro P, Bruzzese N, Anderlini D, Aiossa M, Papacchini M, Campanella L, et al. Development of microbial engineered whole-cell systems for environmental benzene determination. Ecotoxicol Environ Saf. 2011;74:542–9. https://doi.org/10.1016/j.ecoenv.2010.08.006.
Hashimoto Y, Nakamura H, Asaga K, Karube I. A new diagnostic method for soil-borne disease using a microbial biosensor. Microbes Environ. 2008;23:35–9. https://doi.org/10.1264/jsme2.23.35.
Nakamura H, Nakayama Y, Gotoh M. Ferricyanide chronoamperometric total antioxidant capacity assay for green tea. Curr Topic Anal Chem. 2016;10:29–34. http://www.researchtrends.net/tia/abstract.asp?in=0&vn=10tid=30&aid=5937&pub=2016&type=
Kubisch R, Bohrn U, Fleischer M, Stütz E. Cell-based sensor system using l6 cells for broad band continuous pollutant monitoring in aquatic environments. Sensors. 2012;12:3370–93. https://doi.org/10.3390/s120303370.
Nakamura H. Current status of water environment and their microbial biosensor techniques. Part I: Current data of water environment and recent studies on water quality investigations in Japan, and new possibility of microbial biosensor techniques. Anal Bioanal Chem. 2018; https://doi.org/10.1007/s00216-018-0923-z.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no competing interests.
Additional information
Published in the topical collection Microbial Biosensors for Analytical Applications with guest editor Gérald Thouand.
Rights and permissions
About this article
Cite this article
Nakamura, H. Current status of water environment and their microbial biosensor techniques – Part II: Recent trends in microbial biosensor development. Anal Bioanal Chem 410, 3967–3989 (2018). https://doi.org/10.1007/s00216-018-1080-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1080-0