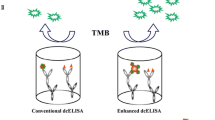
Abstract
Bovine milk is a recognized allergenic food source with β-lactoglobulin (BLG) as its major allergen. Reliable detection of BLG epitopes can, therefore, be a useful marker for the presence of milk in processed food products, and for potential allergenicity. At the present, enzyme-linked immunosorbent assays (ELISA) for the detection of BLG are time-consuming and generally not specific to BLG IgE epitopes. In this study, the 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide-activated anti-BLG IgE epitope monoclonal antibody (mAb 1G9) was covalently bound onto the KOH-treated microtiter plate surface. Using this mAb-bound plate in sandwich combination with biotinylated anti-BLG polyclonal antibody-labeled gold nanoparticles, a linear dynamic range between 31.25 and 64 × 103 ng mL−1 with a limit of detection for BLG of 0.49 ng mL−1 was obtained, which is 32 times wider and 16 times more sensitive than conventional sandwich ELISA (sELISA). Total recovery of BLG in spiked food samples was found, without matrix effects. Also in partially hydrolyzed infant formulas, the allergenic BLG residues were detected quantitatively. Compared with conventional and commercial BLG detection sELISAs, our sELISA is reliable, highly BLG epitope-specific, user-friendly, and time-saving and allows accurate detection of potentially allergenic residues in different types of processed foods. This improved sELISA protocol can be easily extended to detect other well-identified and characterized food allergens.

IgE epitope mAb-bound plate in sandwich combination with gold probe for sensitive and rapid detection of bovine β-lactoglobulin and its potentially allergenic residues




Similar content being viewed by others
Abbreviations
- ALA:
-
Bovine α-lactalbumin
- APTES:
-
3-Aminopropyltriethoxysilane
- AuNPs:
-
Colloidal gold nanoparticles
- BLG:
-
Bovine β-lactoglobulin
- CN:
-
Bovine casein
- EDC:
-
1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide
- mAb:
-
Monoclonal antibody
- MES:
-
2-(N-Morpholino)ethanesulfonic acid
- MW:
-
Molecular weight
- pAb-BLG:
-
Rabbit anti-β-lactoglobulin polyclonal antibody
- PAGE:
-
Polyacrylamide gel electrophoresis
- PBS:
-
Phosphate-buffered saline
- PBST:
-
PBS containing 0.1% Tween-20
- sELISA:
-
Sandwich enzyme-linked immunosorbent assays
- UHT-milk:
-
Ultra heat treated-milk
References
Luyt D, Ball H, Makwana N, Green MR, Bravin K, Nasser SM, et al. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin Exp Allergy. 2014;44:642–72.
Spies J. Milk allergy. J Milk Food Technol. 1973;36:225–31.
Hochwallner H, Schulmeister U, Swoboda I, Spitzauer S, Valenta R. Cow’s milk allergy: from allergens to new forms of diagnosis, therapy and prevention. Methods. 2014;66:22–33.
Taylor SL, Baumert JL, Kruizinga AG, Remington BC, Crevel RW, Brooke-Taylor S, et al. Establishment of reference doses for residues of allergenic foods: report of the VITAL Expert Panel. Food Chem Toxicol. 2014;63:9–17.
Trendelenburg V, Enzian N, Bellach J, Schnadt S, Niggemann B, Beyer K. Detection of relevant amounts of cow’s milk protein in non-pre-packed bakery products sold as cow’s milk-free. Allergy. 2015;70:591–7.
Cucu T, Jacxsens L, De Meulenaer B. Analysis to support allergen risk management: which way to go? J Agric Food Chem. 2013;61:5624–33.
Moreno Mde L, Muñoz-Suano A, López-Casado MÁ, Torres MI, Sousa C, Cebolla Á. Selective capture of most celiac immunogenic peptides from hydrolyzed gluten proteins. Food Chem. 2016;205:36–42.
Zhang H, Lu Y, Ushio H, Shiomi K. Development of sandwich ELISA for detection and quantification of invertebrate major allergen tropomyosin by a monoclonal antibody. Food Chem. 2014;150:151–7.
He SF, Li X, Gao JY, Tong P, Chen HB. Development of a H2O2-sensitive quantum dots-based fluorescent sandwich ELISA for sensitive detection of bovine β-lactoglobulin by monoclonal antibody. J Sci Food Agric. 2018;98:519–26.
He SF, Li X, Gao JY, Tong P, Chen HB. Development of sandwich ELISA for testing bovine beta-lactoglobulin allergenic residues by specific polyclonal antibody against human IgE binding epitopes. Food Chem. 2017;227:33–40.
Vashist SK, Marion Schneider E, Lam E, Hrapovic S, Luong JH. One-step antibody immobilization-based rapid and highly-sensitive sandwich ELISA procedure for potential in vitro diagnostics. Sci Rep. 2014;4:4407.
Vashist SK, Schneider EM, Luong JH. Rapid sandwich ELISA-based in vitro diagnostic procedure for the highly-sensitive detection of human fetuin a. Biosens Bioelectron. 2015;67:73–8.
Li YS, Meng XY, Zhou Y, Zhang YY, Meng XM, Yang L, et al. Magnetic bead and gold nanoparticle probes based immunoassay for beta-casein detection in bovine milk samples. Biosens Bioelectron. 2015;66:559–64.
Ciaurriz P, Fernández F, Tellechea E, Moran JF, Asensio AC. Comparison of four functionalization methods of gold nanoparticles for enhancing the enzyme-linked immunosorbent assay (ELISA). Beilstein J Nanotechnol. 2017;8:244–53.
Järvinen KM, Chatchatee P, Bardina L, Beyer K, Sampson HA. IgE and IgG binding epitopes on α-lactalbumin and β-lactoglobulin in cow’s milk allergy. Int Arch Allergy Immunol. 2001;126:111–8.
de Bievre P, Böttger D, Eastwood C, Hlavay J, Holmgren M, Horwitz W, et al. Eurachem Guide. Thefitness for purpose of analytical methods. A laboratory guide to method validation and related topics: second edition. 2014; http://www.eurachem.ul.pt (accessed July 14, 2017).
Schägger H. Tricine-SDS-PAGE. Nat Protoc. 2006;1:16–22.
de Luis R, Lavilla M, Sanchez L, Calvo M, Perez MD. Development and evaluation of two ELISA formats for the detection of beta-lactoglobulin in model processed and commercial foods. Food Control. 2009;20:643–7.
Badran AA, Morais S, Maquieira Á. Simultaneous determination of four food allergens using compact disc immunoassaying technology. Anal Bioanal Chem. 2017;409:2261–8.
Wu XL, He WY, Ji KM, Wan WP, Hu DS, Wu H, et al. A simple and fast detection method for bovine milk residues in foods: a 2-site monoclonal antibody immunochromatography assay. J Food Sci. 2013;78:M452–7.
Ruiz-Valdepeñas Montiel V, Campuzano S, Conzuelo F, Torrente-Rodríguez RM, Gamella M, Reviejo AJ, et al. Electrochemical magnetoimmunosensing platform for determination of the milk allergen β-lactoglobulin. Talanta. 2015;131:156–62.
Eissa S, Tlili C, L’Hocine L, Zourob M. Electrochemical immunosensor for the milk allergen β-lactoglobulin based on electrografting of organic film on graphene modified screen-printed carbon electrodes. Biosens Bioelectron. 2012;38:308–13.
Wu XL, Li Y, Liu B, Feng Y, He WY, Liu ZG, et al. Two-site antibody immunoanalytical detection of food allergens by surface plasmon resonance. Food Anal Methods. 2016;9:582–8.
Bonfatti V, Grigoletto L, Cecchinato A, Gallo L, Carnier P. Validation of a new reversed-phase high-performance liquid chromatography method for separation and quantification of bovine milk protein genetic variants. J Chromatogr A. 2008;1195:101–6.
Ji J, Zhu P, Pi FW, Sun C, Sun JD, Jia M. Development of a liquid chromatography-tandem mass spectrometry method for simultaneous detection of the main milk allergens. Food Control. 2017;74:79–88.
Ansari P, Stoppacher N, Rudolf J, Schuhmacher R, Baumgartner S. Selection of possible marker peptides for the detection of major ruminant milk proteins in food by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;399:1105–15.
Chung CS, Yamini S, Trumbo PR. FDA’s health claim review: whey-protein partially hydrolyzed infant formula and atopic dermatitis. Pediatrics. 2012;130:e408–14.
Pescuma M, Hébert EM, Rabesona H, Drouet M, Choiset Y, Haertlé T, et al. Proteolytic action of Lactobacillus delbrueckii subsp. bulgaricus CRL 656 reduces antigenic response to bovine β-lactoglobulin. Food Chem. 2011;127:487–92.
Vashist SK, Lam E, Hrapovic S, Male KB, Luong JH. Immobilization of antibodies and enzymes on 3-aminopropyltriethoxysilane-functionalized bioanalytical platforms for biosensors and diagnostics. Chem Rev. 2014;114:11083–130.
Park SJ, Jung WY. Effect of KOH activation on the formation of oxygen structure in activated carbons synthesized from polymeric precursor. J Colloid Interface Sci. 2002;250:93–8.
Ashley J, Piekarska M, Segers C, Trinh L, Rodgers T, Willey R, et al. An SPR based sensor for allergens detection. Biosens Bioelectron. 2017;88:109–13.
Curciarello R, Lareu JF, Fossati CA, Docena GH, Petruccelli S. Immunochemical characterization of Glycine max L. Merr. var Raiden, as a possible hypoallergenic substitute for cow’s milk-allergic patients. Clin Exp Allergy. 2008;38:1559–65.
Candreva ÁM, Smaldini PL, Curciarello R, Fossati CA, Docena GH, Petruccelli S. The major soybean allergen Gly m Bd 28K induces hypersensitivity reactions in mice sensitized to cow’s milk proteins. J Agric Food Chem. 2016;64:1590–9.
Mariager B, S⊘lve M, Eriksen H, Brogren CH. Bovine β-lactoglobulin in hypoallergenic and ordinary infant formulas measured by an indirect competitive ELISA using monoclonal and polyclonal antibodies. Food Agric Immunol. 1994;6:73–83.
Acknowledgments
The work was supported by Research Project of State Key Laboratory of Food Science and Technology (No. SKLF-ZZA-201612, SKLF-ZZB-201712)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All academic authors declare that they have no conflict of interest. Mr. Shandong Wu declares that he is the director of the Hangzhou Zheda Dixum Biological Gene Engineering Co., Ltd., but this company was not financially involved in the present study.
Electronic supplementary material
ESM 1
(PDF 183 kb)
Rights and permissions
About this article
Cite this article
He, S., Li, X., Wu, Y. et al. A novel sandwich enzyme-linked immunosorbent assay with covalently bound monoclonal antibody and gold probe for sensitive and rapid detection of bovine β-lactoglobulin. Anal Bioanal Chem 410, 3693–3703 (2018). https://doi.org/10.1007/s00216-018-1019-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1019-5