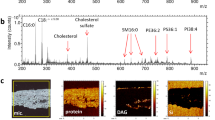
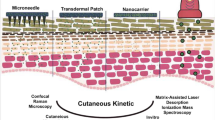
Abstract
Generation of skin distribution profiles and reliable determination of drug molecule concentration in the target region are crucial during the development process of topical products for treatment of skin diseases like psoriasis and atopic dermatitis. Imaging techniques like mass spectrometric imaging (MSI) offer sufficient spatial resolution to generate meaningful distribution profiles of a drug molecule across a skin section. In this study, we use matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) to generate quantitative skin distribution profiles based on tissue extinction coefficient (TEC) determinations of four different molecules in cross sections of human skin explants after topical administration. The four drug molecules: roflumilast, tofacitinib, ruxolitinib, and LEO 29102 have different physicochemical properties. In addition, tofacitinib was administrated in two different formulations. The study reveals that with MALDI-MSI, we were able to observe differences in penetration profiles for both the four drug molecules and the two formulations and thereby demonstrate its applicability as a screening tool when developing a topical drug product. Furthermore, the study reveals that the sensitivity of the MALDI-MSI techniques appears to be inversely correlated to the drug molecules’ ability to bind to the surrounding tissues, which can be estimated by their Log D values.

Graphical abstract






Similar content being viewed by others
References
Wilsmann-Theis D, Hagemann T, Jordan J, Bieber T, Novak N. Facing psoriasis and atopic dermatitis: are there more similarities or more differences? Eur J Dermatology. 2008;18:172–80. https://doi.org/10.1684/ejd.2008.0357.
Schon MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352:1899–912.
Menter A, Gottlieb A, Feldman SR, Van Voorhees AS, Leonardi CL, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–50. https://doi.org/10.1016/j.jaad.2008.02.039.
Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermatovener. 1980;92:44–7.
Lewis-Jones S. Quality of life and childhood atopic dermatitis: the misery of living with childhood eczema. Int J Clin Pract. 2006;60:984–92. https://doi.org/10.1111/j.1742-1241.2006.01047.x.
Ständer S, Steinhoff M. Pathophysiology of pruritus in atopic dermatitis: an overview. Exp Dermatol. 2002;11:12–24. https://doi.org/10.1034/j.1600-0625.2002.110102.x.
Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643–59. https://doi.org/10.1016/j.jaad.2008.12.032.
Thaçi D, Salgo R. Malignancy concerns of topical calcineurin inhibitors for atopic dermatitis: facts and controversies. Clin Dermatol. 2010;28:52–6. https://doi.org/10.1016/j.clindermatol.2009.04.001.
Leite-Silva V, de Almeida M, Fradin A, Grice JE, Roberts MS. Delivery of drugs applied topically to the skin. Expert Rev Dermatol. 2012;7:383–97. https://doi.org/10.1586/edm.12.32.
Feingold KR. Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J Lipid Res. 2007;48:2531–46. https://doi.org/10.1194/jlr.R700013-JLR200.
Michaels AS, Chandrasekaran SK, Shaw JE. Drug permeation through human skin: theory and invitro experimental measurement. AICHE J. 1975;21:985–96. https://doi.org/10.1002/aic.690210522.
Elias PM. Epidermal lipids, barrier function, and desquamation. J Invest Dermatol. 1983;80:44s–9s. https://doi.org/10.1111/1523-1747.ep12537108.
Van Smeden J, Janssens M, Gooris GS, Bouwstra JA. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim Biophys Acta - Mol Cell Biol Lipids. 2014;1841:295–313. https://doi.org/10.1016/j.bbalip.2013.11.00.
Hadgraft J, Lane ME. Skin permeation: the years of enlightenment. Int J Pharm. 2005;305:2–12. https://doi.org/10.1016/j.ijpharm.2005.07.014.
Marks R. The stratum corneum barrier: the final frontier. J Nutr. 2004;134:2017S–21S.
Vickers CH. Existence of reservoir in the stratum corneum: experimental proof. Arch Dermatol. 1963;88:20–3.
Trommer H, Neubert RHH. Overcoming the stratum Corneum: the modulation of skin penetration a review structure of the stratum corneum and drug options to overcome the barrier. Skin Pharmacol Physiol. 2006;19:106–21.
Harrison JE, Watkinson AC, Green DM, Hadgraft J, Brain K. The relative effect of Azone(R) and Transcutol(R) on permeant diffusivity and solubility in human stratum corneum. Pharm Res. 1996;13:542–6. https://doi.org/10.1023/A:1016037803128.
Williams A, Barry B. Penetration enhancers. Adv Drug Deliv Rev. 2012;64:128–37. https://doi.org/10.1016/j.addr.2012.09.032.
Franz TJ. Percutaneous absorption. On the relevance of in vitro data. J Invest Dermatol. 1975;64:190–5. https://doi.org/10.1111/1523-1747.ep12533356.
Kligman AM, Chirstophers E. preparation of isolated sheets of human stratum corneum. Arch Dermatol. 1963;88:702–5.
Lind M, Nielsen KT, Schefe LH, Nørremark K, Eriksson AH, Norsgaard H, et al. Supersaturation of calcipotriene and betamethasone dipropionate in a novel aerosol foam formulation for topical treatment of psoriasis provides enhanced bioavailability of the active ingredients. Dermatol Ther (Heidelb). 2016;6:413–25. https://doi.org/10.1007/s13555-016-0125-6.
Alvarez-Roman R, Naik A, Kalia YN, Fessi H, Guy RH. Visualization of skin penetration using confocal laser scanning microscopy. Eur J Pharm Biopharm. 2004;58:301–16.
Mendelsohn R, Flach CR, Moore DJ. Determination of molecular conformation and permeation in skin via IR spectroscopy, microscopy, and imaging. Biochim Biophys Acta Biomembr. 2006;1758:923–33. https://doi.org/10.1016/j.bbamem.2006.04.009.
Simona Mura, Maria Manconi, Anna Maria Fadda, Maria Chiara Sala, Jacopo Perricc, Elena Pini CS (2012) Penetration enhancer-containing vesicles (PEVs) as carriers for cutaneous delivery of minoxidil: in vitro evaluation of drug permeation by infrared spectroscopy Read More: http://informahealthcare.com/doi/abs/10.3109/10837450.2012.685661. Pharm Dev Technol 18:1–7. https://doi.org/10.3109/10837450.2012.685661
Mao G, Flach CR, Mendelsohn R, Walters RM. Imaging the distribution of sodium dodecyl sulfate in skin by confocal Raman and infrared microspectroscopy. Pharm Res. 2012;29:2189–201.
Freudiger CW, Min W, Saar BG, Lu S, Holtom GR, He C, et al. Label-free biomedical imaging with high sensitivity by stimulated raman scattering microscopy. Science. 2008;322(80):1857–61.
Caprioli RM, Farmer TB, Gile J. Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal Chem. 1997;69:4751–60. https://doi.org/10.1021/Ac970888i.
Bunch J, Clench MR, Richards DS. Determination of pharmaceutical compounds in skin by imageing matrix-assisted laser desorption/ionisation mass spectrometry. Rapid Commun Mass Spectrom. 2004;18:3051–60.
Hart PJ, Francese S, Claude E, Woodroofe MN, Clench MR. MALDI-MS imaging of lipids in ex vivo human skin. Anal Bioanal Chem. 2011;401:115–25. https://doi.org/10.1007/s00216-011-5090-4.
Enthaler B, Pruns JK, Wessel S, Rapp C, Fischer M, Wittern KP. Improved sample preparation for MALDI-MSI of endogenous compounds in skin tissue sections and mapping of exogenous active compounds subsequent to ex-vivo skin penetration. Anal Bioanal Chem. 2012;402:1159–67. https://doi.org/10.1007/s00216-011-5562-6.
Hunt DW, Winters GC, Brownsey RW, Kulpa JE, Gilliland KL, Thiboutot DM, Hofland HE (2017) Inhibition of sebum production with the acetyl coenzyme a carboxylase inhibitor olumacostat glasaretil. J Invest Dermatol. https://doi.org/10.1016/j.jid.2016.12.031.
Sorensen IS, Janfelt C, Nielsen MMB, Mortensen RW, Knudsen NO, Eriksson AH, et al. Combination of MALDI-MSI and cassette dosing for evaluation of drug distribution in human skin explant. Anal Bioanal Chem. 2017;409:4993–5005. https://doi.org/10.1007/s00216-017-0443-2.
Judd AM, Scurr DJ, Heylings JR, Wan KW, Moss GP. Distribution and visualisation of chlorhexidine within the skin using ToF-SIMS: a potential platform for the design of more efficacious skin antiseptic formulations. Pharm Res. 2013;30:1896–905. https://doi.org/10.1007/s11095-013-1032-5.
Čižinauskas V, Elie N, Brunelle A, Briedis V. Fatty acids penetration into human skin ex vivo : a TOF-SIMS analysis approach. Biointerphases. 2017;12:11003. https://doi.org/10.1116/1.4977941.
Sjövall P, Greve TM, Clausen SK, Moller K, Eirefelt S, Johansson B, et al. Imaging of distribution of topically applied drug molecules in mouse skin by combination of time-of-flight secondary ion mass spectrometry and scanning electron microscopy. Anal Chem. 2014;86:3443–52. https://doi.org/10.1021/ac403924w.
Stauber J. Quantitation by MS imaging: needs and challenges in pharmaceuticals. Bioanalysis. 2012;4:2095–8.
Pirman DA, Reich RF, Kiss A, Heeren RMA, Yost RA. Quantitative MALDI tandem mass spectrometric imaging of cocaine from brain tissue with a deuterated internal standard. Anal Chem. 2013;85:1081–9. https://doi.org/10.1021/ac302960j.
Turker SD, Dunn WB, Wilkie J. MALDI MS of drugs: profiling, imaging and steps towards quantitative analysis. Appl Spectrosc Rev. 2016;4928:00–0. https://doi.org/10.1080/05704928.2016.1207659.
Takai N, Tanaka Y, Watanabe A, Saji H. Quantitative imaging of a therapeutic peptide in biological tissue sections by MALDI MS. Bioanalysis. 2013;5:603–12. https://doi.org/10.4155/bio.13.13.
Hamm G, Bonnel D, Legouffe R, Pamelard F, Delbos JM, Bouzom F, et al. Quantitative mass spectrometry imaging of propranolol and olanzapine using tissue extinction calculation as normalization factor. J Proteome. 2012;75:4952–61. https://doi.org/10.1016/j.jprot.2012.07.035.
Stoeckli M, Staab D, Schweitzer A. Compound and metabolite distribution measured by MALDI mass spectrometric imaging in whole-body tissue sections. Int J Mass Spectrom. 2007;260:195–202. https://doi.org/10.1016/j.ijms.2006.10.007.
Felding J, Sørensen MD, Poulsen TD, Larsen J, Andersson C, Refer P, et al. Discovery and early clinical development of 2-{6-[2-(3,5-Dichloro-4-pyridyl)acetyl]-2,3-dimethoxyphenoxy}-N-propylacetamide (LEO 29102), a soft-drug inhibitor of phosphodiesterase 4 for topical treatment of atopic dermatitis. J Med Chem. 2014;57:5893–903.
Boswell-Smith V, Spina D. PDE4 inhibitors as potential therapeutic agents in the treatment of COPD-focus on roflumilast. Int J Chron Obs Pulmon Dis. 2007;2:121–9.
Garnock-Jones KP. Roflumilast: a review in COPD. Drugs. 2015;75:1645–56. https://doi.org/10.1007/s40265-015-0463-1.
Tofacitinib. Drugs R D. 2010;10:271–84.
Bachelez H, Van De Kerkhof PCM, Strohal R, Kubanov A, Valenzuela F, Lee JH, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet. 2015;386:552–61. https://doi.org/10.1016/S0140-6736(14)62113-9.
Mesa RA, Yasothan U, Kirkpatrick P. Ruxolitinib. Nat Rev Drug Discov. 2012;11(2):103–4. https://doi.org/10.1038/nrd3652.
Kofoed K, Skov L, Zachariae C. New drugs and treatment targets in psoriasis. Acta Derm Venereol. 2015;95:133–9. https://doi.org/10.2340/00015555-1931.
Magnusson BM, Anissimov YG, Cross SE, Roberts MS. Molecular size as the main determinant of solute maximum flux across the skin. J Invest Dermatol. 2004;122:993–9. https://doi.org/10.1111/j.0022-202X.2004.22413.x.
Potts RO, Guy RH. A predictive algorithm for skin permeability: the effects of molecular size and hydrogen bond activity. Pharm Res An Off J Am Assoc Pharm Sci. 1995;12:1628–33. https://doi.org/10.1023/A:1016236932339.
Magnusson BM, Cross SE, Winckle G, Roberts MS. Percutaneous absorption of steroids: determination of in vitro permeability and tissue reservoir characteristics in human skin layers. Skin Pharmacol Physiol. 2006;19:336–42. https://doi.org/10.1159/000095254.
Zhang Q, Li P, Roberts MS. Maximum transepidermal flux for similar size phenolic compounds is enhanced by solvent uptake into the skin. J Control Release. 2011;154:50–7. https://doi.org/10.1016/j.jconrel.2011.04.018.
Zhang Q, Li P, Liu D, Roberts MS. Effect of vehicles on the maximum transepidermal flux of similar size phenolic compounds. Pharm Res. 2012;30:1–9. https://doi.org/10.1007/s11095-012-0846-x.
Sanagi MM, Ling SL, Nasir Z, Hermawan D, Wan Ibrahim WA, Naim AA. Comparison of signal-to-noise, blank determination, and linear regression methods for the estimation of detection and quantification limits for volatile organic compounds by gas chromatography. J AOAC Int. 2009;92:1833–8.
Laznicek M, Laznickova A. The effect of lipophilicity on the protein binding and blood cell uptake of some acidic drugs. J Pharm Biomed Anal. 1995;13:823–8.
Acknowledgements
We acknowledge Tina Leonhardt Hjort for the execution of the drug penetration study, Liselotte Saustrup Kirk for the UHPLC-MS/MS analysis of the tissue samples, and Lotte Koue for the help with the production of the formulations. We also acknowledge Torsten Kåre Askland, Edma Edge Fontaine, and Mathieu Gaudin for helping with the proofreading.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
The study involved anonymized human skin samples from abdominoplasty surgery acquired from Biopredic International, France, upon written informed consent from the donor.
Ethical approval
According to the Danish Committee Act section 14.3, anonymous human biological material used in health research projects shall not seek ethical approval as long as the collected human biological material has been collected lawfully in the country of origin which is the case. The supplier of the human biological material holds a permit granted by the French Ministry of Higher Education and Research for the acquisition, transformation, sales, and export of human biological material to be used in research, and is furthermore in compliance with the French law CSP1245-2.
Electronic supplementary material
ESM 1
(PDF 220 kb)
Rights and permissions
About this article
Cite this article
Bonnel, D., Legouffe, R., Eriksson, A.H. et al. MALDI imaging facilitates new topical drug development process by determining quantitative skin distribution profiles. Anal Bioanal Chem 410, 2815–2828 (2018). https://doi.org/10.1007/s00216-018-0964-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-0964-3