Abstract
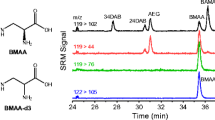
We describe a set of new tools for the detection and quantification of β-N-methylamino-l-alanine (BMAA) which includes a novel stable isotope-labeled BMAA standard (13C3,15N2) and a chip-based capillary electrophoresis mass spectrometry platform for separation and detection. Baseline resolution of BMAA from its potentially confounding structural isomers N-2-aminoethylglycine (AEG) and 2,4-diaminobutyric acid (2,4-DAB) is achieved using the chip-based CE-MS system in less than 1 min. Detection and linearity of response are demonstrated across > 3.5 orders of dynamic range using parallel reaction monitoring (PRM). The lower limit of detection and quantification were calculated for BMAA detection at 40 nM (4.8 ng/mL) and 400 nM (48 ng/mL), respectively. Finally, the strategy was applied to detect BMAA in seafood samples purchased at a local market in Raleigh, NC where their harvest location was known. BMAA was detected in a sea scallop sample. Because the BMAA/stable isotope-labeled 13C3,15N2-BMAA (SIL-BMAA) ratio in the scallop sample was below the limit of quantification, a semiquantitative analysis of BMAA content was carried out, and BMAA content was estimated to be approximately 820 ng BMAA/1 g of wet scallop tissue. Identification was verified by high mass measurement accuracy of precursor (< 5 ppm) and product ions (< 10 ppm), comigration with SIL-BMAA spike-in standard, and conservation of ion abundance ratios for product ions between BMAA and SIL-BMAA. Interestingly, BMAA was not identified in the free protein fraction but only detected after protein hydrolysis which suggests that BMAA is tightly bound by and/or incorporated into proteins.

Utilization of novel 13C3,15N2-BMAA and chip-based CE-MS/MS for detection and quantification of BMAA in environmental samples.




Similar content being viewed by others
References
Vega A, Bell EA. Alpha-amino-beta-methylaminopropionic acid a new amino acid from seeds of Cycas circinalis. Phytochemistry. 1967;6(5):759. https://doi.org/10.1016/S0031-9422(00)86018-5.
Spencer PS, Nunn PB, Hugon J, Ludolph AC, Ross SM, Roy DN, et al. Guam amyotrophic-lateral-sclerosis parkinsonism dementia linked to a plant excitant neurotoxin. Science. 1987;237(4814):517–22. https://doi.org/10.1126/science.3603037.
Bradley WG, Mash DC. Beyond Guam: the cyanobacteria/BMAA hypothesis of the cause of ALS and other neurodegenerative diseases. Amyotrophic Lateral Scler. 2009;10(Suppl 2):7–20. https://doi.org/10.3109/17482960903286009.
Reed D, Labarthe D, Chen KM, Stallones R. A cohort study of amyotrophic-lateral-sclerosis and parkinsonism-dementia on Guam and Rota. Am J Epidemiol. 1987;125(1):92–100.
Cox PA, Banack SA, Murch SJ. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. P Natl Acad Sci USA. 2003;100(23):13380–3. https://doi.org/10.1073/pnas.2235808100.
Cox PA, Sacks OW. Cycad neurotoxins, consumption of flying foxes, and ALS-PDC disease in Guam. Neurology. 2002;58(6):956–9.
Banack SA, Cox PA. Biomagnification of cycad neurotoxins in flying foxes—implications for ALS-PDC in Guam. Neurology. 2003;61(3):387–9.
Cox PA, Banack SA, Murch SJ, Rasmussen U, Tien G, Bidigare RR, et al. Diverse taxa of cyanobacteria produce beta-N-methylamino-L-alanine, a neurotoxic amino acid. Proc Natl Acad Sci U S A. 2005;102(14):5074–8. https://doi.org/10.1073/pnas.0501526102.
Hallegraeff GM. A review of harmful algal blooms and their apparent global increase. Phycologia. 1993;32(2):79–99. https://doi.org/10.2216/i0031-8884-32-2-79.1.
Jackson JBC. Ecological extinction and evolution in the brave new ocean. P Natl Acad Sci USA. 2008;105:11458–65. https://doi.org/10.1073/pnas.0802812105.
Caller TA, Doolin JW, Haney JF, Murby AJ, West KG, Farrar HE, et al. A cluster of amyotrophic lateral sclerosis in New Hampshire: a possible role for toxic cyanobacteria blooms. Amyotrophic Lateral Scler. 2009;10(Suppl 2):101–8. https://doi.org/10.3109/17482960903278485.
Stommel EW, Field NC, Caller TA. Aerosolization of cyanobacteria as a risk factor for amyotrophic lateral sclerosis. Med Hypotheses. 2013;80(2):142–5. https://doi.org/10.1016/j.mehy.2012.11.012.
Metcalf JS, Codd GA. Cyanobacteria, neurotoxins and water resources: are there implications for human neurodegenerative disease? Amyotrophic Lateral Scler. 2009;10:74–8. https://doi.org/10.3109/17482960903272942.
Jiang LY, Kiselova N, Rosen J, Ilag LL. Quantification of neurotoxin BMAA (beta-N-methylamino-L-alanine) in seafood from Swedish markets. Sci Rep-Uk. 2014;4 https://doi.org/10.1038/srep06931.
Holtcamp W. Shark fin consumption may expose people to neurotoxic BMAA. Environ Health Persp. 2012;120(5):A191-A.
Brand LE, Pablo J, Compton A, Hammerschlag N, Mash DC. Cyanobacterial blooms and the occurrence of the neurotoxin, beta-N-methylamino-l-alanine (BMAA), in South Florida aquatic food webs. Harmful Algae. 2010;9(6):620–35. https://doi.org/10.1016/j.hal.2010.05.002.
Cox PA, Davis DA, Mash DC, Metcalf JS, Banack SA. Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. P Roy Soc B-Biol Sci. 2016;283(1823) https://doi.org/10.1098/rspb.2015.2397.
Frøyset AK, Khan EA, Fladmark KE. Quantitative proteomics analysis of zebrafish exposed to sub-lethal dosages of β-methyl-amino-L-alanine (BMAA). Sci Rep. 2016;6 https://doi.org/10.1038/srep29631.
Beri J, Nash T, Martin RM, Bereman MS. Exposure to BMAA mirrors molecular processes linked to neurodegenerative disease. Proteomics. 2017;17(17–18). doi:https://doi.org/10.1002/pmic.201700161.
Faassen EJ. Presence of the neurotoxin BMAA in aquatic ecosystems: what do we really know? Toxins. 2014;6(3):1109–38. https://doi.org/10.3390/toxins6031109.
Chernoff N, Hill DJ, Diggs DL, Faison BD, Francis BM, Lang JR, et al. A critical review of the postulated role of the non-essential amino acid, beta-N-methylamino-L-alanine, in neurodegenerative disease in humans. J Toxicol Env Heal B. 2017;20(4):183–229. https://doi.org/10.1080/10937404.2017.1297592.
Eriksson J, Jonasson S, Papaefthimiou D, Rasmussen U, Bergman B. Improving derivatization efficiency of BMAA utilizing AccQ-tag(a (R)) in a complex cyanobacterial matrix. Amino Acids. 2009;36(1):43–8. https://doi.org/10.1007/s00726-007-0023-4.
Pan M, Mabry TJ, Cao P, Moini M. Identification of nonprotein amino acids from cycad seeds as N-ethoxycarbonyl ethyl ester derivatives by positive chemical-ionization gas chromatography mass spectrometry. J Chromatogr A. 1997;787(1–2):288–94. https://doi.org/10.1016/S0021-9673(97)00789-9.
Guo T, Geis S, Hedman C, Arndt M, Krick W, Sonzogni W. Characterization of ethyl chloroformate derivative of beta-methylamino-L-alanine. J Am Soc Mass Spectr. 2007;18(5):817–25. https://doi.org/10.1016/j.jasms.2007.01.006.
Roy-Lachapelle A, Solliec M, Sauve S. Determination of BMAA and three alkaloid cyanotoxins in lake water using dansyl chloride derivatization and high-resolution mass spectrometry. Anal Bioanal Chem. 2015;407(18):5487–501. https://doi.org/10.1007/s00216-015-8722-2.
Rosen J, Hellenas KE. Determination of the neurotoxin BMAA (beta-N-methylamino-L-alanine) in cycad seed and cyanobacteria by LC-MS/MS (liquid chromatography tandem mass spectrometry). Analyst. 2008;133(12):1785–9. https://doi.org/10.1039/b809231a.
Combes A, El Abdellaoui S, Sarazin C, Vial J, Mejean A, Ploux O, et al. Validation of the analytical procedure for the determination of the neurotoxin beta-N-methylamino-L-alanine in complex environmental samples. Anal Chim Acta. 2013;771:42–9. https://doi.org/10.1016/j.aca.2013.02.016.
Jiang LY, Aigret B, De Borggraeve WM, Spacil Z, Ilag LL. Selective LC-MS/MS method for the identification of BMAA from its isomers in biological samples. Anal Bioanal Chem. 2012;403(6):1719–30. https://doi.org/10.1007/s00216-012-5966-y.
Banack SA, Metcalf JS, Spacil Z, Downing TG, Downing S, Long A, et al. Distinguishing the cyanobacterial neurotoxin beta-N-methylamino-L-alanine (BMAA) from other diamino acids. Toxicon. 2011;57(5):730–8. https://doi.org/10.1016/j.toxicon.2011.02.005.
Kerrin ES, White RL, Quilliam MA. Quantitative determination of the neurotoxin beta-N-methylamino-L-alanine (BMAA) by capillary electrophoresis-tandem mass spectrometry. Anal Bioanal Chem. 2017;409(6):1481–91. https://doi.org/10.1007/s00216-016-0091-y.
Sharma V, Eckels J, Taylor GK, Shulman NJ, Stergachis AB, Joyner SA, et al. Panorama: a targeted proteomics knowledge base. J Proteome Res. 2014;13(9):4205–10. https://doi.org/10.1021/pr5006636.
Reveillon D, Abadie E, Sechet V, Masseret E, Hess P, Amzil Z. Beta-N-methylamino-L-alanine (BMAA) and isomers: distribution in different food web compartments of Thau lagoon, French Mediterranean Sea. Mar Environ Res. 2015;110:8–18. https://doi.org/10.1016/j.marenvres.2015.07.015.
Dunlop RA, Cox PA, Banack SA, Rodgers KJ. The non-protein amino acid BMAA is misincorporated into human proteins in place of L-serine causing protein misfolding and aggregation. Plos One. 2013;8(9) https://doi.org/10.1371/journal.pone.0075376.
Rosen J, Westerberg E, Schmiedt S, Hellenas KE. BMAA detected as neither free nor protein bound amino acid in blue mussels. Toxicon. 2016;109:45–50. https://doi.org/10.1016/j.toxicon.2015.11.008.
Faassen E, Antoniou M, Beekman-Lukassen W, Blahova L, Chernova E, Christophoridis C, et al. A collaborative evaluation of LC-MS/MS based methods for BMAA analysis: soluble bound BMAA found to be an important fraction. Marine Drugs. 2016;14(3):45.
Jiang LY, Eriksson J, Lage S, Jonasson S, Shams S, Mehine M, et al. Diatoms: a novel source for the neurotoxin BMAA in aquatic environments. Plos One. 2014;9(1) https://doi.org/10.1371/journal.pone.0084578.
Reveillon D, Sechet V, Hess P, Amzil Z. Production of BMAA and DAB by diatoms (Phaeodactylum tricornutum, Chaetoceros sp., Chaetoceros calcitrans and, Thalassiosira pseudonana) and bacteria isolated from a diatom culture. Harmful Algae. 2016;58:45–50. https://doi.org/10.1016/j.hal.2016.07.008.
Acknowledgements
All of the mass spectrometry measurements were carried out in the Molecular Education, Technology, and Research Innovation Center (METRIC) at NC State University.
Funding
David C. Muddiman would like to acknowledge the NC State University Chancellor’s Innovation Fund (CIF). Michael S. Bereman would like to acknowledge NC State University for startup funding and the Center for Human Health and the Environment (CHHE), P30ES025128.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This research did not involve human participants or animals.
Conflicts of interest
David C. Muddiman is named on a patent that is pending for which Cambridge Isotope Laboratories pays royalties to NC State University. None of the remaining authors have any conflicts of interest.
Electronic supplementary material
ESM 1
(PDF 264 kb)
Rights and permissions
About this article
Cite this article
Beri, J., Kirkwood, K.I., Muddiman, D.C. et al. A novel integrated strategy for the detection and quantification of the neurotoxin β-N-methylamino-l-alanine in environmental samples. Anal Bioanal Chem 410, 2597–2605 (2018). https://doi.org/10.1007/s00216-018-0930-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-0930-0