Abstract
This manuscript reports on the application of chemometric methods for the development of an optimized microfluidic paper-based analytical device (μPAD). As an example, we applied chemometric methods for both device optimization and data processing of results of a colorimetric uric acid assay. Box–Behnken designs (BBD) were utilized for the optimization of the device geometry and the amount of thermal inkjet-deposited assay reagents, which affect the assay performance. Measurement outliers were detected in real time by partial least squares discriminant analysis (PLS-DA) of scanned images. The colorimetric assay mechanism is based on the on-device formation of silver nanoparticles (AgNPs) through the interaction of uric acid, ammonia, and poly(vinyl alcohol) with silver ions under mild basic conditions. The yellow color originating from visible light absorption by localized surface plasmon resonance of AgNPs can be detected by the naked eye or, more quantitatively, with a simple flat-bed scanner. Under optimized conditions, the linearity of the calibration curve ranges from 0.1–5 × 10−3 mol L−1 of uric acid with a limit of detection of 33.9 × 10−6 mol L−1 and a relative standard of deviation 4.5% (n = 3 for determination of 5.0 × 10−3 mol L−1 uric acid).

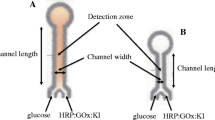
A chemometrics-assisted microfluidic paper-based analytical device was developed as a low-cost and rapid platform for the determination of uric acid (UA). The detection method is based on the chemical interaction of UA, ammonia, and polyvinyl alcohol under mild basic condition with silver ions inducing formation of yellow silver nanoparticles (AgNPs).





Similar content being viewed by others
References
Martinez AW, Phillips ST, Butte MJ, Whitesides GM. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew Chem Int Ed. 2007;46:1318–20.
Cate DM, Adkins JA, Mettakoonpitak J, Henry CS. Recent developments in paper-based microfluidic devices. Anal Chem. 2015;87:19–41.
Yamada K, Shibata H, Suzuki K, Citterio D. Toward practical application of paper-based microfluidics for medical diagnostics: state-of-the-art and challenges. Lab Chip. 2017;17:1206–49.
Carrilho E, Martinez AW, Whitesides GM. Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal Chem. 2009;81:7091–5.
Yamada K, Henares TG, Suzuki K, Citterio D. Paper-based inkjet-printed microfluidic analytical devices. Angew Chem Int Ed. 2015;54:5294–310.
Czitrom V. One-factor-at-a-time versus designed experiments. Am Stat. 1999;53:126–31.
Jalali-Heravi M, Arrastia M, Gomez FA. How can chemometrics improve microfluidic research? Anal Chem. 2015;87:3544–55.
Ferreira SLC, Bruns RE, da Silva EGP, dos Santos WNL, Quintella CM, David JM, et al. Statistical designs and response surface techniques for the optimization of chromatographic systems. J Chromatogr A. 2007;1158:2–14.
Pumtang S, Siripornnoppakhun W, Sukwattanasinitt M, Ajavakom A. Solvent colorimetric paper-based polydiacetylene sensors from diacetylene lipids. J Colloid Interf Sci. 2011;364:366–72.
Villa JEL, Poppi RJ. A portable SERS method for the determination of uric acid using a paper-based substrate and multivariate curve resolution. Analyst. 2016;141:1966–72.
Shariati-Rad M, Irandoust M, Mohammadi S. Multivariate analysis of digital images of a paper sensor by partial least squares for determination of nitrite. Chemometr Intell Lab. 2016;158:48–53.
Avoundjian A, Jalali-Heravi M, Gomez FA. Use of chemometrics to optimize a glucose assay on a paper microfluidic platform. Anal Bioanal Chem. 2017;409:2697–703.
Zolgharnein J, Shahmoradi A, Ghasemi JB. Comparative study of Box–Behnken, central composite, and Doehlert matrix for multivariate optimization of Pb (II) adsorption onto Robinia tree leaves: multivariate optimization. J Chemometr. 2013;27:12–20.
Box GEP, Behnken DW. Some new three level designs for the study of quantitative variables. Technometrics. 1960;2:455–75.
Bezerra MA, Santelli RE, Oliveira EP, Villar LS, Escaleira LA. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76:965–77.
Jun S, Irudayaraj J, Demirci A, Geiser D. Pulsed UV-light treatment of corn meal for inactivation of Aspergillus niger spores. Int J Food Sci Tech. 2003;38:883–8.
Popa E, Kubota Y, Tryk DA, Fujishima A. Selective voltammetric and amperometric detection of uric acid with oxidized diamond film electrodes. Anal Chem. 2000;72:1724–7.
Pradhan T, Maiti S, Kumar R, Lee YH, Kim JW, Lee JH, et al. Rationally designed non-enzymatic fluorogenic ‘turn-on’ probe for uric acid. Dyes Pigments. 2015;121:1–6.
Jain PK, Huang X, El-Sayed IH, El-Sayed MA. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res. 2008;41:1578–86.
Cobley CM, Skrabalak SE, Campbell DJ, Xia Y. Shape-controlled synthesis of silver nanoparticles for plasmonic and sensing applications. Plasmonics. 2009;4:171–9.
Mock JJ, Barbic M, Smith DR, Schultz DA, Schultz S. Shape effects in plasmon resonance of individual colloidal silver nanoparticles. J Chem Phys. 2002;116:6755–9.
Amjadi M, Rahimpour E. Silver nanoparticles plasmon resonance-based method for the determination of uric acid in human plasma and urine samples. Microchim Acta. 2012;178:373–9.
Bera RK, Anoop A, Raj CR. Enzyme-free colorimetric assay of serum uric acid. Chem Commun. 2011;47:11498–500.
Li X, Liu X. Fabrication of three-dimensional microfluidic channels in a single layer of cellulose paper. Microfluid Nanofluid. 2014;16:819–27.
Zhao C, Thuo MM, Liu X. A microfluidic paper-based electrochemical biosensor array for multiplexed detection of metabolic biomarkers. Sci Technol Adv Mat. 2013;14:054402.
Heil, W, Ehrhardt, V. Reference ranges for adults and children: pre-analytical considerations. 9th ed. Roche Diagnostics GmbH; 2008. https://www.rochediagnostics.fr/htdocs/media/pdf/actualites/2a_reference_ranges_2008.pdf. Accessed 8 May 2017.
Martinez AW, Phillips ST, Carrilho E, Thomas SW, Sindi H, Whitesides GM. Simple telemedicine for developing regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal Chem. 2008;80:3699–707.
Wu LP, Li YF, Huang CZ, Zhang Q. Visual detection of Sudan dyes based on the plasmon resonance light scattering signals of silver nanoparticles. Anal Chem. 2006;78:5570–7.
Sharma VK, Yngard RA, Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interfac. 2009;145:83–96.
Yin Y, Li Z-Y, Zhong Z, Gates B, Xia Y, Venkateswaran S. Synthesis and characterization of stable aqueous dispersions of silver nanoparticles through the Tollens process. J Mater Chem. 2002;12:522–7.
Pourreza N, Golmohammadi H, Naghdi T, Yousefi H. Green in-situ synthesized silver nanoparticles embedded in bacterial cellulose nanopaper as a bionanocomposite plasmonic sensor. Biosens Bioelectron. 2015;74:353–9.
Sampat R, Young S, Rosen A, Bernhard D, Millington D, Factor S, et al. Potential mechanisms for low uric acid in Parkinson disease. J Neural Transm. 2016;123:365–70.
Cardoso VF, Martins P, Botelho G, Rebouta L, Lanceros-Méndez S, Minas G. Degradation studies of transparent conductive electrodes on electroactive poly(vinylidene fluoride) for uric acid measurements. Sci Technol Adv Mat. 2010;11:045006.
Song Z-H, Hou S. Chemiluminescence assay for uric acid in human serum and urine using flow-injection with immobilized reagents technology. Anal Bioanal Chem. 2002;372:327–32.
Cai W, Lai J, Lai T, Xie H, Ye J. Controlled functionalization of flexible graphene fibers for the simultaneous determination of ascorbic acid, dopamine and uric acid. Sensor Actuat B Chem. 2016;224:225–32.
Li X-L, Shi Q, Jin W, Li G, Todoroki K, Mizuno H, Toyo’oka T, Min JZ. Uric acid quantification in fingernail of gout patients and healthy volunteers using HPLC-UV: quantitative assessment of uric acid in fingernail of gout patients. Biomed Chromatogr. 2016;1338-1342.
Dai X, Fang X, Zhang C, Xu R, Xu B. Determination of serum uric acid using high-performance liquid chromatography (HPLC)/isotope dilution mass spectrometry (ID-MS) as a candidate reference method. J Chromatogr B. 2007;857:287–95.
Jin D, Seo M-H, Huy BT, Pham Q-T, Conte ML, Thangadurai D, et al. Quantitative determination of uric acid using CdTe nanoparticles as fluorescence probes. Biosens Bioelectron. 2016;77:359–65.
Kumar A, Hens A, Arun RK, Chatterjee M, Mahato K, Layek K, et al. A paper based microfluidic device for easy detection of uric acid using positively charged gold nanoparticles. Analyst. 2015;140:1817–21.
Acknowledgments
We acknowledge professor Lutgarde M.C. Buydens (Radboud University, Nijmegen, the Netherlands) for her help and advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent of the participant who provided the urine sample was obtained.
Electronic supplementary material
ESM 1
(PDF 201 kb)
Rights and permissions
About this article
Cite this article
Hamedpour, V., Postma, G.J., van den Heuvel, E. et al. Chemometrics-assisted microfluidic paper-based analytical device for the determination of uric acid by silver nanoparticle plasmon resonance. Anal Bioanal Chem 410, 2305–2313 (2018). https://doi.org/10.1007/s00216-018-0879-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-0879-z