Abstract
The format of an immunochromatographic multiassay is first proposed with native antisera and a universal conjugate of antispecies antibodies with gold nanoparticles. This format allows (1) the exclusion of purification and conjugation stages for specific antibodies and (2) significant reduction of the concentration of specific antibodies in the system. The independent use of specific antibodies and a conjugated marker provided a low detection limit and high signal intensity. The proposed format was implemented for the simultaneous detection of two herbicides. The instrumental limits for the detection of atrazine and chlorsulfuron were 0.1 and 0.7 ng/mL, respectively, and the analysis time was 20 min. The suitability of the test system for monitoring these herbicides in nontreated apple and blackcurrant juices is shown. The assay technique is simple, sensitive, and easily transferrable to any other antigen.

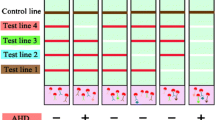
The proposed format of the immunochromatographic multiassay is based on the use of native antisera and a universal conjugate of antispecies antibodies with gold nanoparticles. In this way purification and conjugation stages for specific antibodies are excluded, and the concentrations of specific antibodies and the conjugated marker can be varied independently to obtain a low detection limit.





Similar content being viewed by others
References
Posthuma-Trumpie G, Korf J, van Amerongen A. Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem. 2009;393(2):569–82.
Wong RC, Tse HY. Lateral flow immunoassay. New York: Humana; 2009. p. 224.
Dzantiev BB, Byzova NA, Urusov AE, Zherdev AV. Immunochromatographic methods in food analysis. Trends Anal Chem. 2014;55:81–93.
Chandler J, Gurmin T, Robinson N. The place of gold in rapid tests. IVD Technol. 2000;6:37–49.
Ivanova JL, Edelweiss EF, Leonova OG, Balandin TG, Popenko VI, Deyev SM. Application of fusion protein 4D5 scFv-dibarnase: barstar–gold complex for studying P185 HER2 receptor distribution in human cancer cells. Biochimie. 2012;94(8):1833–6.
Byzova NA, Zherdev AV, Sveshnikov PG, Sadykhov EG, Dzantiev BB. Development of an immunochromatographic test system for the detection of Helicobacter pylori antigens. Appl BiochemMicrobiol. 2015;51(5):608–17.
Bradbury A, Plückthun A. Reproducibility: standardize antibodies used in research. Nature. 2015;518:27–9.
Polakiewicz RD. Antibodies: the solution is validation. Nature. 2015;518(7540):483.
Weller MG. Quality issues of research antibodies. Anal Chem Insights. 2016;11:21–7.
Josic D, Lim Y-P. Analytical and preparative methods for purification of antibodies. Food Technol Biotechnol. 2001;39(3):215–26.
Urusov AE, Zherdev AV, Dzantiev BB. Use of gold nanoparticle-labeled secondary antibodies to improve the sensitivity of an immunochromatographic assay for aflatoxin B1. Microchim Acta. 2014;181(15-16):1939–46.
Urusov AE, Petrakova AV, Zherdev AV, Dzantiev BB. “Multistage in one touch” design with a universal labeling conjugate for high-sensitive lateral flow immunoassays. Biosens Bioelectron. 2016;86:575–9.
Burgos NR, Tranel PJ, Streibig JC, Davis VM, Shaner D, Norsworthy JK, et al. Review: confirmation of resistance to herbicides and evaluation of resistance levels. Weed Sci. 2013;61(1):4–20.
Mudhoo A, Garg VK. Sorption, transport and transformation of atrazine in soils, minerals and composts: a review. Pedosphere. 2011;21(1):11–25.
Boffetta P, Adami HO, Berry SC, Mandel JS. Atrazine and cancer: a review of the epidemiologic evidence. Eur J Cancer Prev. 2013;22(2):169–80.
Yazynina EV, Zherdev AV, Dzantiev BB, Izumrudov VA, Gee SJ, Hammock BD. Microplate immunoassay technique using polyelectrolyte carriers: kinetic studies and application to detection of the herbicide atrazine. Anal Chim Acta. 1999;399(1):151–60.
Yazynina EV, Zherdev AV, Eremin SA, Popova VA, Dzantiev BB. Development of enzyme immunoassays for the herbicide chlorsulfuron. Appl BiochemMicrobiol. 2002;38(1):9–14.
Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci. 1973;241(105):20–2.
Byzova NA, Zvereva EA, Zherdev AV, Eremin SA, Dzantiev BB. Rapid pretreatment-free immunochromatographic assay of chloramphenicol in milk. Talanta. 2010;81(3):843–8.
Hermanson GT. Preparation of colloidal gold-labeled proteins. In: Hermanson GT, editor. Bioconjugate techniques. 3rd ed. Boston: Academic; 2013. p. 924–35.
Sotnikov DV, Byzova NA, Zherdev AV, Eskendirova SZ, Baltin KK, Mukanov KK, et al. Express immunochromatographic detection of antibodies against Brucella abortus in cattle sera based on quantitative photometric registration and modulated cut-off level. J Immunoassay Immunochem. 2015;36(1):80–90.
Urusov AE, Kostenko SN, Sveshnikov PG, Zherdev AV, Dzantiev BB. Ochratoxin A immunoassay with surface plasmon resonance registration: lowering limit of detection by the use of colloidal gold immunoconjugates. Sens Actuators B. 2011;156(1):343–9.
Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104(1):293–346.
Wang S, Quan Y, Lee N, Kennedy IR. Rapid determination of fumonisin B1 in food samples by enzyme-linked immunosorbent assay and colloidal gold immunoassay. J Agric Food Chem. 2006;54(7):2491–5.
Yang H, Li D, He R, Guo Q, Wang K, Zhang X, et al. A novel quantum dots-based point of care test for syphilis. Nanoscale Res Lett. 2010;5(5):875–81.
Chuanlai X, Huting W, Chifang P, Zhengyu J, Liqiang L. Colloidal gold-based immumochromatographic assay for detection of diethylstilbestrol residues. Biomed Chromatogr. 2006;20(12):1390–4.
Kaur J, Singh KV, Boro R, Thampi KR, Raje M, Varshney GC, et al. Immunochromatographic dipstick assay format using gold nanoparticles labeled protein−hapten conjugate for the detection of atrazine. Envir Sci Technol. 2007;41(14):5028–36.
Byzova NA, Zherdev AV, Zvereva EA, Dzantiev BB. Immunochromatographic assay with photometric detection for rapid determination of the herbicide atrazine and other triazines in foodstuffs. J AOAC Int. 2010;93(1):36–43.
Miocevic O, Cole CR, Laughlin MJ, Buck RL, Slowey PD, Shirtcliff EA. Quantitative lateral flow assays for salivary biomarker assessment: a review. Front Public Health. 2017;5:133.
Masiri J, Benoit L, Thienes C, Kainrath C, Barrios-Lopez B, Agapov A, et al. A rapid, semi-quantitative test for detection of raw and cooked horse meat residues. Food Control. 2017;76:102–7.
Galan-Malo P, Lopez M, Ortiz JC, Perez MD, Sanchez L, Razquin P, et al. Detection of egg and milk residues on working surfaces by ELISA and lateral flow immunoassay tests. Food Control. 2017;74:45–53.
Li Y, Liu L, Song S, Kuang H. Development of a gold nanoparticle immunochromatographic assay for the on-site analysis of 6-benzylaminopurine residues in bean sprouts. Food Agric Immunol. 2017; https://doi.org/10.1080/09540105.2017.1354359.
Wang Z, Zheng Q, Guo L, Suryoprabowo S, Liu L, Kuang H. Preparation of an anti-dexamethasone monoclonal antibody and its use in development of a colloidal gold immunoassay. Food AgricImmunol. 2017;28(6):958–68.
Liang Q, Yi C, Jiang L, Tan G, Zhang C, Wang B. Development of a lateral flow dipstick immunoassay for evaluation of folate levels in maize. Anal Bioanal Chem. 2017;409(24):5655–60.
Goryacheva IY, Lenain P, De Saeger S. Nanosized labels for rapid immunotests. Trends Anal Chem. 2013;46:30–43.
Gong X, Cai J, Zhang B, Zhao Q, Piao J, Peng W, et al. A review of fluorescent signal-based lateral flow immunochromatographic strips. J Mater Chem B. 2017;5(26):5079–91.
Paterson AS, Raja B, Mandadi V, Townsend B, Lee M, Buel A, et al. A low-cost smartphone-based platform for highly sensitive point-of-care testing with persistent luminescent phosphors. Lab Chip. 2017;17(6):1051–9.
Connolly R, O'Kennedy R. Magnetic lateral flow immunoassay test strip development – Considerations for proof of concept evaluation. Methods. 2017;116:132–40.
Sanchez-Purra M, Roig-Solvas B, Versiani A, Rodriguez-Quijada C, de Puig H, Bosch I, et al. Design of SERS nanotags for multiplexed lateral flow immunoassays. Mol Syst Des Eng. 2017;2(4):401–9.
Wang L, Qian C, Qian W, Wang R, Wu J, Ying Y. A highly specific strategy for in suit detection of DNA with nicking enzyme assisted amplification and lateral flow. Sens Actuators B. 2017;253:258–65.
Zhong Y, Chen Y, Yao L, Zhao D, Zheng L, Liu G, et al. Gold nanoparticles based lateral flow immunoassay with largely amplified sensitivity for rapid melamine screening. Microchim Acta. 2016;183(6):1989–94.
Rodriguez MO, Covian LB, Garcia AC, Blanco-Lopez MC. Silver and gold enhancement methods for lateral flow immunoassays. Talanta. 2016;148:272–8.
Cho IH, Bhunia A, Irudayaraj J. Rapid pathogen detection by lateral-flow immunochromatographic assay with gold nanoparticle-assisted enzyme signal amplification. Int J Food Microbiol. 2015;206:60–6.
Acknowledgements
The authors are thankful to S.M. Pridvorova (A.N. Bach Institute of Biochemistry) for the transmission electron microscopy measurements and data processing. This research was financially supported by the Russian Science Foundation (grant 14-16-00149).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Research involving human participants and/or animals
The studies did not involve human participants or animals.
Electronic supplementary material
ESM 1
(PDF 463 kb)
Rights and permissions
About this article
Cite this article
Byzova, N.A., Urusov, A.E., Zherdev, A.V. et al. Multiplex highly sensitive immunochromatographic assay based on the use of nonprocessed antisera. Anal Bioanal Chem 410, 1903–1910 (2018). https://doi.org/10.1007/s00216-018-0853-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-0853-9