Abstract
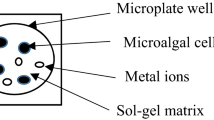
Optical biosensors for the detection of toxic species in aqueous media were developed via the encapsulation of microalgae in sol–gel matrices. In a first step, the effect of cadmium(II), lead(II), and anthracene on the chlorophyll a fluorescence intensity of Anabaena flos-aquae, Chlorella vulgaris, and Euglena gracilis microalgae in suspension was studied. Complementary ATP-metry measurements demonstrated a direct relationship between optical response and pollutant toxicity, in a cell- and dose-dependent manner. In a second step, microalgae were successfully encapsulated in silicate–colloidal silica nanocomposite matrices. However, a complete loss of cell response to pollutant addition was observed, despite the preservation of cell viability. Introduction of a low amount (5 mol%) of amine- or ethyl-bearing silanes in the matrix formulation allowed the recovery of the sensing capacity of the immobilized microalgae, without impacting on the response time (30 s). Porosimetry and 29Si solid-state NMR spectroscopy showed that the organic moieties are fully integrated into the inorganic network, tuning the ability of the target pollutant to diffuse and reach the encapsulated algae. This versatile strategy could be useful for the easy and fast assessment of contamination levels in polluted waters.

Microalgal biosensors for aqueous contaminants using organically doped silica as cellular hosts







Similar content being viewed by others
References
Torres MA, Barros MP, Campos SCG, Pinto E, Rajamani S, Sayre RT, et al. Biochemical biomarkers in algae and marine pollution. Ecotox Environ Safe. 2008;71:1–15.
Trang PTK, Berg M, Viet PH, Mui NV, van der Meer JR. Bacterial bioassay for rapid and accurate analysis of arsenic in highly variable groundwater samples. Environ Sci Technol. 2005;39:7625–30.
Augusto J, Brenneman TB. Assessing systemicity of peanut fungicides through bioassay of plant tissues with Sclerotium rolfsii. Plant Dis. 2012;96:330–7.
Avishai N, Rabinowitz C, Moiseeva E, Rinkevich B. Genotoxicity of the Kishon River, Israel: the application of an in vitro cellular assay. Mutat Res. 2002;518:21–37.
Durrieu C, Tran-Minh C, Chovelon JM, Barthet L, Chouteau C, Védrine C. Algal biosensors for aquatic ecosystems monitoring. Eur Phys J Appl Phys. 2006;36:205–9.
Scognamiglio V, Pezzotti G, Pezzotti I, Cano J, Buonasera K, Giannini D, et al. Biosensors for effective environmental and agrifood protection and commercialization: from research to market. Microchim Acta. 2010;170:215–25.
Brayner R, Couté A, Livage J, Perrette C. Sicard, C Micro-algal biosensors. Anal Bioanal Chem. 2011;401:581–97.
Monteiro CM, Castro PML, Malcata FX. Metal uptake by microalgae: underlying mechanisms and practical applications. Biotechnol Prog. 2012;28:299–311.
Sánchez-Rodrıguez I, Huerta-Diaz MA, Choumiline E, Holguın-Quinones O, Zertuche-González JA. Elemental concentrations in different species of seaweeds from Loreto Bay, Baja California Sur, Mexico: implications for the geochemical control of metals in algal tissue. Environ Poll. 2001;114:145–60.
Gill I, Ballesteros A. Bioencapsulation within synthetic polymers (part 2): non-sol-gel protein-polymer biocomposites. Trends Biotechnol. 2000;18:469–79.
Depagne C, Roux C, Coradin T. How to design cell-based biosensors using the sol-gel process. Anal Bional Chem. 2011;400:965–76.
Moreno-Garrido I. Microalgae immobilization: current techniques and uses. Bioresour Technol. 2008;99:3949–64.
Böttcher H, Soltmann U, Mertig M, Pompe W. Biocers: ceramics with incorporated microorganisms for biocatalytic, biosorptive and functional materials development. J Mater Chem. 2004;14:2176–88.
Rooke JC, Leonard A, Meunier CF, Su BL. Designing photobioreactors based on living cells immobilized in silica gel for carbon dioxide mitigation. ChemSusChem. 2011;4:1249–57.
Blondeau M, Coradin T. Living materials from sol–gel chemistry: current challenges and perspectives. J Mater Chem. 2012;22:22335–43.
Dickson DJ, Ely RL. Silica sol-gel encapsulation of cyanobacteria: lessons for academic and applied research. Appl Microbiol Biotechnol. 2013;97:1809–19.
Pena-Vazquez E, Maneiro E, Perez-Conde C, Moreno-Bondi MC, Costas E. Microalgae fiber optic biosensors for herbicide monitoring using sol–gel technology. Biosens Bioelectron. 2009;24:3538–43.
Darder M, Aranda P, Burgos-Asperilla L, Llobera A, Cadarso VJ, Fernandez-Sanchez C, et al. Algae–silica systems as functional hybrid materials. J Mater Chem. 2010;20:9362–9.
Perullini M, Ferro Y, Durrieu C, Jobbagy M, Bilmes SA. Sol–gel silica platforms for microalgae-based optical biosensors. J Biotechnol. 2014;179:65–70.
Pannier A, Soltmann U, Altenburger R, Schmitt-Jansen M. Alginate/silica hybrid materials for immobilization of green microalgae Chlorella vulgaris for cell-based sensor arrays. J Mater Chem B. 2014;2:7896–910.
Nassif N, Bouvet O, Rager MN, Roux C, Coradin T, Livage J. Living bacteria in silica gels. Nat Mater. 2002;1:42–4.
Dahoumane SA, Yéprémian C, Djédiat C, Couté A, Fiévet F, Coradin T, et al. Improvement of kinetics, yield, and colloidal stability of biogenic gold nanoparticles using living cells of Euglena gracilis microalga. J Nanopart Res. 2016;18:79.
Järup L, Akesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238:201–8.
Flora G, Gupta D, Towari A. Toxicity of lead: a review with recent updates. Interdiscip Toxicol. 2012;5:47–58.
Sverdrup LE, Krogh PH, Nielsen T, Kjaer C, Stenersen J. Toxicity of eight polycyclic aromatic compounds to red clover (Trifolium pratense), ryegrass (Lolium perenne), and mustard (Sinapsis alba). Chemosphere. 2003;53:993–1003.
Crouch SP, Kozlowski R, Slater KJ, Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immun Methods. 1993;160:81–8.
Blondeau M, Brayner R, Guyot F, Coradin T. Correlating biological methods to assess Escherichia coli bacteria viability in silica gels. Anal Methods. 2014;6:2429–31.
Fung M, Khitrin AK, Ermolaev K. An improved broadband decoupling sequence for liquid crystals and solids. J Magn Reson. 2000;142:97–101.
Lesage A, Sakellariou D, Hediger S, Elena B, Charmont P, Steuernagel S, et al. Experimental aspects of proton NMR spectroscopy in solids using phase-modulated homonuclear dipolar decoupling. J Magn Reson. 2003;163:105–13.
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl Chem. 2015;87:1051–69.
Lassiaz S, Labarre D, Galarneau A, Brunel D, Mutin PH. Modification of silica by an organic monolayer in aqueous medium using octylphosphonic acid and aluminium species. J Mater Chem. 2011;21:8199–205.
Bernardoni F, Fadeev AY. Adsoption and wetting characterization of hydrophobic SBA-15 silica. J Colloid Interface Sci. 2011;356:690–8.
Brunel D, Cauvel A, Di Renzo F, Fajula F, Fubini B, Onida B, et al. Preferential grafting of alkoxysilane coupling agents on the hydrophobic portion of the surface of micell-templated silica. New J Chem. 2000;24:807–13.
Bonhomme C, Coelho C, Baccile N, Gervais C, Azaïs T, Babonneau F. Advanced solid state NMR techniques for the characterization of sol-gel derived materials. Acc Chem Res. 2007;40:738–46.
Gervais C, Coelho C, Azaïs T, Maquet J, Laurent G, Pourpoint F, et al. First principles NMR calculations of phenylphosphinic acid C6H5HPO(OH): assignments, orientation of tensors by local field experiments and effect of molecular motion. J Magn Reson. 2007;187:131–40.
Heng LY, Jusoh K, Ling CHM, Idris M. Toxicity of single and combinations of lead and cadmium to the cyanobacteria Anabaena flos-aquae. Bull Environ Contam Toxicol. 2004;72:373–9.
Surosz W, Palinska KA. Effects of heavy-metal stress on cyanobacterium Anabaena flos-aquae. Arch Environ Contamin Toxicol. 2004;48:40–8.
Bajguz A. Suppression of Chlorella vulgaris growth by cadmium, lead, and copper stress and its restoration by endogenous brassinolide. Arch Environ Contamin Toxicol. 2011;60:406–16.
Ouyang HL, Kong XZ, He W, Qin N, He QS, Wang Y, et al. Effects of five heavy metals at sub-lethal concentrations on the growth and photosynthesis of Chlorella vulgaris. Chin Sci Bull. 2012;57:3363–70.
Navarro L, Torres-Marquez ML, Gonzalez-Moreno S, Devars S, Hernandez R, Moreno-Sanchez R. A comparison of physiological changes in the protist Euglena gracilis during exposure to heavy metals of heterotrophic and autotrophic cells. Comp Biochem Physiol. 1997;116:265–72.
McGrath JA, Di Toro DM. Validation of the target lipid model for toxicity assessment of residual petroleum constituents : monocyclic and polycyclic aromatic hydrocarbons. Environ Toxicol Chem. 2009;28:1130–48.
Aksmann A, Tukaj Z. The Effect of anthracene and phenanthrene on the growth, photosynthesis, and SOD activity of the green alga Scenedesmus armatus depends on the PAR irradiance and CO2 level. Arch Environ Contam Toxicol. 2004;47:177–84.
Patel JG, Nirmal Kumar JI, Khan SR. Consequences of environmentally hazardous polycyclic aromatic hydrocarbon- anthracene treatment on cyanobacteria. Int J Appl Sci Biotechnol. 2015;3:381–6.
Dugger DL, Stanton JH, Irby BN, McConnell BL, Cummings WW, Maatman RW. The exchange of twenty metal ions with the weakly acidic silanol group of silica gel. J Phys Chem. 1964;68:757–60.
Nassif N, Roux C, Coradin T, Bouvet OMM, Livage J. Bacteria quorum sensing in silica matrices. J Mater Chem. 2004;14:2264–8.
Nassif N, Roux C, Rager MN, Bouvet OMM, Coradin T, Livage J. A sol-gel matrix to preserve the viability of encapsulated bacteria. J Mater Chem. 2003;13:203–8.
Aguado J, Arsuaga JM, Arencibia A, Lindo M, Gascon V. Aqueous heavy metals removal by adsorption on amine-functionalized mesoporous silica. J Hazard Mater. 2009;163:213–21.
Acknowledgements
N.B.’s PhD studies were funded by the French Ministry for Superior Education and Research. The authors thank G. Thouand (GEnie des Procédés Environnement - Agroalimentaire (GEPEA), Université de Nantes) and B. Lebeau (Institut des Sciences des Matériaux de Mulhouse (ISMM), Université de Haute Alsace) for fruitful discussions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Published in the topical collection Microbial Biosensors for Analytical Applications with guest editor Gérald Thouand.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 3049 kb)
Rights and permissions
About this article
Cite this article
Ahmed, N.B., Masse, S., Laurent, G. et al. Optical microalgal biosensors for aqueous contaminants using organically doped silica as cellular hosts. Anal Bioanal Chem 410, 1205–1216 (2018). https://doi.org/10.1007/s00216-017-0405-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0405-8