Abstract
Electrogenerated chemiluminescence (ECL) reactions between tris(2,2′-bipyridine)ruthenium(II) and PAMAM dendrimers of the full (G1.0) and half (G1.5) generations were carried out in an aqueous medium at pH 6.1 and 10.0. In the absence of 5-fluoro-1H,3H-pyrimidine-2,4-dione (5-fluorouracil, 5-Fu) (coreactant effect study), the ECL efficiency trends as a function of [G1.0] and [G1.5] at pH 6.1 and 10.0 revealed that PAMAM dendrimers are about 100 (G1.5, pH 6.1), 60 (G1.5, pH 10.0), 26 (G1.0, pH 10.0) and 13 (G1.0, pH 6.1) times more efficient as ECL coreactants than oxalate anion is. Moreover, ECL reactions were done in the presence of several solutions of 5-Fu at a fixed concentration of the G1.0 and G1.5 dendrimers at pH 6.1 and 10.0 (binding study). The ECL efficiency trends as a function of [5-Fu] highlighted a dendrimer/5-Fu binding. Therefore, one of the most remarkable and novel findings of this work is the potential of PAMAM dendrimers to be used as both sensors and biosensors in an aqueous medium in the presence of a suitable sensitizer. Redox potentials of the [Ru(bpy)3]3+/2+ couple were also determined in the absence and presence of 5-Fu at both pHs. In the absence of 5-Fu the positive or negative shift of redox potentials showed the influence of the repulsive or attractive electrostatic long-range and short-range interactions between the charged dendrimer surface and the oxidized and reduced forms of the couple. In the presence of 5-Fu the trends of redox potentials highlighted the existence of a charged dendrimer/5-Fu species.

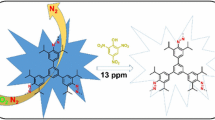
ECL emission for the [Ru(bpy)3]2+/ G1.0 dendrimer reaction in the presence of the 5-Fu at pH 6.1






Similar content being viewed by others
Notes
CV experiments and ECL emissions were recorded simultaneously from a Potentiostat /Galvanostat (Biologic SP-50) synchronized to a PTI fluorescence spectrometer. A Peltier system interfaced to the instrument set was employed to maintain a constant temperature of 298.15 ± 0.01 K inside the spectro-electrochemical cell. The reading and handling of voltammograms and ECL emissions was done with a PC using EC-Lab Express and Felix32 software, respectively. The software allowed for the subtraction of the files and the integration of both current–time and ECL intensity–time curves.
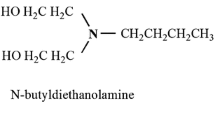
Given that the end groups of the dendrimers (–COO− for G1.5 and –NH2 for G1.0) are responsible for the ECL effect we assume that the reaction ECL mechanisms are similar to those corresponding to [Ru(bpy)3]2+ + C2O4 2− and [Ru(bpy)3]2+ + TPA in aqueous solutions (see, e.g., [9, 16]). In the case of the first reaction, the first electron transfer corresponds to a catalytic reaction, Ru(bpy)3 3+ + C2O4 2− ⇌ Ru(bpy)3 2+ + CO2 •− + CO2, and the second one produces Ru(II)*, Ru(bpy)3 3+ + CO2 •− → Ru(bpy)3 2+* + CO2.
The electrostatic repulsion between the positively charged Ru(II) species and the electric field arising from the surface of the G1.0 dendrimer at pH 6.1 can be accounted for by studying the shift of the redox potentials of the [Ru(bpy)3]3+/2+ couple. In this case, E peak − E 0 = (RT/F) ln(γox /γred), where E peak and E 0 are the peak and standard potentials, respectively, γox and γred refer to the activity coefficients of the oxidized and reduced species of the pair, and RT/F have the usual meaning. In the presence of a positively charged dendrimer surface both γox and γred increase as a result of destabilization of both the oxidized and reduced forms of the couple [Ru(bpy)3]3+/2+, but the increase in γox will be greater than the one corresponding to γred as a result of the [Ru(bpy)3]3+ form bearing a higher positive charge. This effect results in a positive shift of redox potentials.
In fact, we have determined the redox potentials of 5-Fu in the presence of Ru(II) complex and in their absence, the values obtained being 1.372 and 1.426 V vs. SHE, respectively. It should be noted that both redox processes were practically not reversible; thus, the previous values refer to the anodic wave peaks.
References
Richter MM. Electrochemiluminescence (ECL). Chem Rev. 2004;104(6):3003–36.
Miao W. Electrogenerated chemiluminescence and its biorelated applications. Chem Rev. 2008;108(7):2506–53.
Hu L, Xu G. Applications and trends in electrochemiluminescence. Chem Soc Rev. 2010;39(8):3275–304.
Liu X, Shi L, Niu W, Li H, Xu G. Environmentally friendly and highly sensitive ruthenium(II) tris(2,2′-bipyridyl) electrochemiluminescent system using 2-(dibutylamino) ethanol as co-reactant. Angew Chem Int Ed. 2007;46(3):421–4.
Zheng L, Wang B, Chi Y, Song S, Fan C, Chen G. Using stannous ion as an excellent inorganic ECL coreactant for tris(2,2′-bipyridyl) ruthenium(II). Dalton Trans. 2012;41(5):1630–4.
Joshi T, Barbante GJ, Francis PS, Hogan CF, Bond AM, Spiccia L. Electrochemiluminescent peptide nucleic acid-like monomers containing Ru(II)-dipyridoquinoxaline and Ru(II)-dipyridophenazine complexes. Inorg Chem. 2011;50(23):12172–83.
Joshi T, Barbante GJ, Francis PS, Hogan CF, Bond AM, Gasser G, et al. Electrochemiluminescent monomers for solid support syntheses of Ru(II)-PNA bioconjugates: multimodal biosensing tools with enhanced duplex stability. Inorg Chem. 2012;51(5):3302–15.
Rubinstein I, Bard AJ. Electrogenerated chemiluminescence. Aqueous ECL systems based on Ru(2,2′-bipyridine)3 2+ and oxalate or organic acids. J Am Chem Soc. 1981;103(3):512–6.
Kanoufi F, Bard AJ. Electrogenerated chemiluminescence. 65. An investigation of the oxidation of oxalate by tris(polypyridine) ruthenium complexes and the effect of the electrochemical steps on the emission intensity. J Phys Chem B. 1999;103(47):10469–80.
Forster RJ, Bertoncello P, Keyes TE. Electrogenerated chemiluminescence. Annu Rev Anal Chem. 2009;2:359–85.
Kerr E, Doeven EH, Barbante GJ, Hogan CF, Bower DJ, Donnelly PS, et al. Annihilation electrogenerated chemiluminescence of mixed metal chelates in solution: modulating emission colour by manipulating the energetics. Chem Sci. 2015;6(1):472–9.
Stringer BD, Quan LM, Barnard PJ, Wilson DJD, Hogan CF. Iridium complexes of N-heterocyclic carbene ligands: investigation into the energetic requirements for efficient electrogenerated chemiluminescence. Organometallics. 2014;33(18):4860–72.
Ma D-L, Chan DS-H, Leung C-H. Organometallic compounds for therapeutic and bioanalytical applications. Acc Chem Res. 2014;47(12):3614–31.
Xiao F-N, Wang M, Wang F-B, Xia X-H. Graphene-ruthenium(II) complex composites for sensitive ECL immunosensors. Small. 2014;10(4):706–16.
Lu L, Chan DS-H, Kwong DWJ, He H-Z, Leung C-H, Ma D-L. Detection of nicking endonuclease activity using a G-quadruplex-selective luminescent switch-on probe. Chem Sci. 2014;5(12):4561–8.
Barbante GJ, Kebede N, Hindson CM, Doeven EH, Zammit EM, Hanson GR, et al. Control of excitation and quenching in multi-colour electrogenerated chemiluminescence systems through choice of co-reactant. Chem Eur J. 2014;20(43):14026–31.
Kebede N, Francis PS, Barbante GJ, Hogan CF. Electrogenerated chemiluminescence of tris(2,2′-bipyridine)ruthenium(II) using common biological buffers as co-reactant, pH buffer and supporting electrolyte. Analyst. 2015;140(21):7142–5.
Perez-Tejeda P, Prado-Gotor R, Grueso EM. Electrochemiluminescence of the [Ru(bpy)3]2+ complex: the coreactant effect of PAMAM dendrimers in an aqueous medium. Inorg Chem. 2012;51(20):10825–31.
Jimenez-Ruiz A, Grueso E, Perez-Tejeda P. Electrogenerated chemiluminescence reactions between the [Ru(bpy)3]2+ complex and PAMAM GX.0 dendrimers in an aqueous medium. J Inorg Biochem. 2015;151:18–25.
Carter MT, Bard AJ. Electrochemical investigations of the interaction of metal chelates with DNA. Electrogenerated chemiluminescent investigation of the interaction of tris(1,10-phenanthroline)ruthenium(II) with DNA. Bioconjug Chem. 1990;1(4):257–63.
Rodriguez M, Bard AJ. Electrochemical studies of the interaction of metal chelates with DNA. 4. Voltammetric and electrogenerated chemiluminescent studies of the interaction of tris(2,2′-bipyridine)osmium(II) with DNA. Anal Chem. 1990;62(24):2658–62.
Astruc D, Boisselier E, Ornelas C. Dendrimers designed for functions: from physical, photophysical, and supramolecular properties to applications in sensing, catalysis, molecular electronics, photonics, and nanomedicine. Chem Rev. 2010;110(4):1857–959.
Frost T, Margerum LD. Effect of PAMAM dendrimers on *Ru(bpy)3 2+ emission quenching by ferrocyanide and on ANS fluorescence: quantitative binding parameters as a function of dendrimer size, pH, and buffer composition. Macromolecules. 2010;43(3):1218–26.
Gopidas KR, Leheny AR, Caminati G, Turro NJ, Tomalia DA. Photophysical investigation of similarities between starburst dendrimers and anionic micelles. J Am Chem Soc. 1991;113(19):7335–42.
Tomalia DA, Naylor AM, Goddard III WA. Starbust dendrimers: molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew Chem Int Ed. 1990;29(2):138–75.
Campagna S, Denti G, Serroni S, Juris A, Venturi M, Ricevuto V, et al. Dendrimers of nanometer-size based on metal-complexes: luminiscent and redox-active polynuclear metal-complexes containing up to 22 metal centers. Chem Eur J. 1995;1(4):211–21.
Stone DL, Smith DK, McGrail PT. Ferrocene encapsulated within symmetric dendrimers: a deeper understanding of dendritic effects on redox potential. J Am Chem Soc. 2002;124(5):856–64.
Beezer AE, King ASH, Martin IK, Mitchel JC, Twyman LJ, Wain CF. Dendrimers as potential drug carriers; encapsulation of acidic hydrophobes within water soluble PAMAM derivatives. Tetrahedron. 2003;59(22):3873–80.
Bhadra D, Bhadra S, Jain S, Jain NK. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int J Pharm. 2003;257(1-2):111–24.
Mintzer MA, Dane EL, O’Toole GA, Grinstaff MW. Exploiting dendrimer multivalency to combat emerging and re-emerging infectious diseases. Mol Pharm. 2012;9(3):342–54.
Kirkpatrick GJ, Plumb JA, Sutcliffe OB, Flint DJ, Wheate NJ. Evaluation of anionic half generation 3.5-6.5 poly(amidoamine) dendrimers as delivery vehicles for the active component of the anticancer drug cisplatin. J Inorg Biochem. 2011;105(9):1115–22.
Pisani MJ, Wheate NJ, Keene FR, Aldrich-Wright JR, Collins JG. Anionic PAMAM dendrimers as drug delivery vehicles for transition metal-based anticancer drugs. J Inorg Biochem. 2009;103(3):373–80.
Tekade RK, Kumar PV, Jain NK. Dendrimers in oncology: an expanding horizon. Chem Rev. 2009;109(1):49–87.
Molla MR, Rangadurai P, Pavan GM, Thayumanavan S. Experimental and theoretical investigations in stimuli responsive dendrimer-based assemblies. Nanoscale. 2015;7(9):3817–37.
Jang YH, Sowers LC, Çagin T, Goddard III WA. First principles calculation of pKa values for 5-substituted uracils. J Phys Chem A. 2001;105:274–80.
Niu YH, Sun L, Crooks RM. Determination of the intrinsic proton binding constants for poly(amidoamine) dendrimers via potentiometric pH titration. Macromolecules. 2003;36(15):5725–31.
Van Duijvenbode RC, Rajanayagam A, Koper GJM, Baars MWPL, De Waal BFM, Meijer EW, et al. Synthesis and protonation behavior of carboxylate-functionalized poly(propyleneimine) dendrimers. Macromolecules. 2000;33(1):46–52.
Gui G-F, Zhuo Y, Chai Y-Q, Xiang Y, Yuan R. A novel ECL biosensor for β-lactamase detection: using Ru(II) linked-ampicillin complex as the recognition element. Biosens Bioelectron. 2015;70:221–5.
Xu Y, Zhang L, Liu Y, Jin Z, Zhao Q, Yang F, et al. Sensitive and selective determination of GSH based on the ECL quenching of Ru(II)1,10-phenanthroline-5,6-dione complex. Biosens Bioelectron. 2016;77:182–7.
Leung K-H, He H-Z, He B, Zhong H-J, Lin S, Wang Y-T, et al. Label-free luminescence switch-on detection of hepatitis C virus NS3 helicase activity using a G-quadruplex-selective probe. Chem Sci. 2015;6(4):2166–71.
Lin S, Gao W, Tian Z, Yang C, Lu L, Mergny J-L, et al. Luminescence switch-on detection of protein tyrosine kinase-7 using a G-quadruplex-selective probe. Chem Sci. 2015;6(7):4284–90.
Staffilani M, Höss E, Giesen U, Schneider E, Hartl FE, Josel H-PP, et al. Multimetallic ruthenium(II) complexes as electrochemiluminescent labels. Inorg Chem. 2003;42(24):7789–98.
Zhou M, Roovers J. Dendritic supramolecular assembly with multiple Ru(II)tris(bipyridine) units at the periphery: synthesis, spectroscopic, and electrochemical study. Macromolecules. 2001;34(2):244–52.
Lee D-N, Park H-S, Kim E-H, Jun Y-M, Lee J-Y, Lee W-Y, et al. Synthesis of novel electrochemiluminescent polyamine dendrimers functionalized with polypyridyl Ru(II) complexes and their electrochemical properties. Bull Kor Chem Soc. 2006;27(1):99–105.
Lee DN, Kim JK, Park HS, Jun YM, Hwang RY, Lee W-YY, et al. Polyamidoamine dendrimers functionalized with electrochemiluminescent polypyridyl Ru(II) complexes. Synth Met. 2005;150(1):93–100.
Sun F, Chen F, Fei W, Sun L, Wu Y. A novel strategy for constructing electrochemiluminescence sensor based on CdS-polyamidoamine incorporating electrodeposited gold nanoparticle film and its application. Bull Kor Chem Soc. 2012;166–167:702–7.
Lu C, Wang X-F, Xu J-J, Chen H-Y. Electrochemical modulation of electrogenerated chemiluminescence of CdS nano-composite. Electrochem Commun. 2008;10(10):1530–2.
Jie G, Yuan J, Zhang J. Quantum dots-based multifunctional dendritic superstructure for amplified electrochemiluminescence detection of ATP. Biosens Bioelectron. 2012;31(1):69–76.
Jie G, Yuan J, Huang T, Zhao Y. Electrochemiluminescence of dendritic magnetic quantum dots nanostructure and its quenching by gold nanoparticles for cancer cells assay. Electroanalysis. 2012;24(5):1220–5.
Jie G, Wang L, Yuan J, Zhang S. Versatile electrochemiluminescence assays for cancer cells based on dendrimer/CdSe-ZnS-quantum dot nanoclusters. Anal Chem. 2011;83(10):3873–80.
Venkatanarayanan A, Crowley K, Lestini E, Keyes TE, Rusling JF, Forster RJ. High sensitivity carbon nanotube based electrochemiluminescence sensor array. Biosens Bioelectron. 2012;31(1):233–9.
Ma F, Zhang Y, Qi H, Gao Q, Zhang C, Miao W. Ultrasensitive electrogenerated chemiluminescence biosensor for the determination of mercury ion incorporating G4 PAMAM dendrimer and Hg(II)-specific oligonucleotide. Biosens Bioelectron. 2012;32(1):37–42.
Yuan Y, Gan X, Chai Y, Yuan R. A novel electrochemiluminescence aptasensor based on in situ generated proline and matrix polyamidoamine dendrimers as coreactants for signal amplication. Biosens Bioelectron. 2014;55:313–7.
Fan F-RF. Experimental techniques of electrogenerated chemiluminescence. In: Bard AJ, editor. Electrogenerated chemiluminescence. New York: Dekker; 2004. p. 23–99.
Mussell RD, Nocera DG. Effect of long-distance electron transfer on chemiluminescence efficiencies. J Am Chem Soc. 1988;110(9):2764–72.
Sanchez-Burgos F, Galan M, Dominguez M, Perez-Tejeda P. Medium effects on the electron transfer transition within the binuclear complex [(NH3)5RuIII-NC-RuII(CN)5]-. New J Chem. 1998;22(8):907–11.
D’Emanuele A, Attwood D. Dendrimer-drug interactions. Adv Drug Deliv Rev. 2005;57(15):2147–62.
Jin Y, Ren X, Wang W, Ke L, Ning E, Du L, et al. A 5-fluorouracil-loaded pH-responsive dendrimer nanocarrier for tumor targeting. Int J Pharm. 2011;420(2):378–84.
Acknowledgements
This work was financed by the Consejería de Educación y Ciencia de la Junta de Andalucía (FQM-03623).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
Additional information
Published in the topical collection Analytical Electrochemiluminescence with guest editors Hua Cui, Francesco Paolucci, Neso Sojic, and Guobao Xu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 121 kb)
Rights and permissions
About this article
Cite this article
Jimenez-Ruiz, A., Grueso, E., Perez-Tejeda, P. et al. Electrochemiluminescent (ECL) [Ru(bpy)3]2+/PAMAM dendrimer reactions: coreactant effect and 5-fluorouracil/dendrimer complex formation. Anal Bioanal Chem 408, 7213–7224 (2016). https://doi.org/10.1007/s00216-016-9816-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9816-1