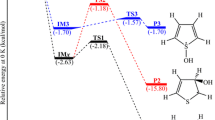
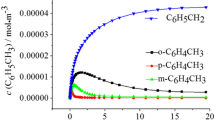
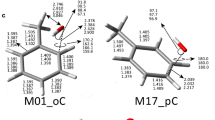
Abstract
The reaction of toluene (T) with ·OH produces addition products as well as the benzyl radical (TR). TR can react with ·OH or O2 to produce oxygenated species, for many of which there is no experimental information available. We present here theoretically determined heats of formation (HFs) of 17 such species using the non-isodesmic reactions on the potential energy surface of TR + O2 and T + ·OH +O2. For those species the experimental HFs of which are known, we obtained a good correlation between experimental and theoretical values at the G4 (r2 = 0.999) and M06/cc-pVQZ (r2 = 0.997) levels, thus showing the goodness of the methods used. Experimentally unknown HFs of other radicals (benzyloxyl, spiro [1,2-dioxetane benzyl], hydroxyphenyl and benzylperoxyl) and closed-shell species (salicylic alcohol, benzo[b]oxetane and p-hydroxy cyclohexa-2,5-dienone) were later determined using those methods.




Similar content being viewed by others
References
Atkinson R (1990) Gas-phase tropospheric chemistry of organic compounds: a review. Atmos Environ 24A:1–41
Atkinson R (1985) Kinetics and mechanisms of the gas-phase reactions of the hydroxyl radical with organic compounds under atmospheric conditions. Chem Rev 85:69–201
Yang Y, Shao M, Wang X, Nolscher AC, Kessel S, Guenther A, Williams J (2016) Towards a quantitative understanding of total OH reactivity: a review. Atmos Environ 134:147–161
Nayebzadeh M, Vahedpour M (2017) A review on reactions of polycyclic aromatic hydrocarbons with the most abundant atmospheric chemical fragments: theoretical and experimental data. Prof React Kinet Mech 42:201–220
Metcalfe WK, Dooley S, Dryer FI (2011) Comprehensive detailed chemical kinetic modeling study of toluene oxidation. Energy Fuels 25:4915–4936
Baltaretu CO, Lichtman EI, Hadler AB, Elrod MJ (2009) Primary atmospheric oxidation mechanism for toluene. J Phys Chem A 113:221–230
Hatipoglu A, Vione D, Yalçin Y, Minero C, Ҫinar Z (2010) Photo-oxidative degradation of toluene in aqueous media by hydroxyl radicals. J Photochem Photobiol A Chem 215:59–68
Wu R, Pan S, Li Y, Wang L (2014) Atmospheric oxidation mechanism of toluene. J Phys Chem A 118:4533–4547
Ji Y, Zhao J, Terazono H, Misawa K, Levitt NP, Li Y, Lin Y, Peng Y, Wang Y, Duan L, Pan B, Zhang F, Feng X, An T, Marrero-Ortiz W, Secrest J, Zhang AL, Shibuya K, Molina MJ, Zhang R (2017) Reassesing the atmospheric oxidation mechanism of toluene. Proc Natl Acad Sci 114:8169–8174
Murakami Y, Oguchi T, Hashimoto K, Nosaka Y (2007) Theoretical study of the benzyl + O2 reaction: kinetics, mechanism, and product branching ratios. J Phys Chem A 111:13200–13208
Bounaceur R, da Costa I, Fournet R, Billaud F, Battin-Leclerc F (2005) Experimental and modeling study of the oxidation of toluene. Chem Kinet 37:25–49
Salta Z, Kosmas AM, Segovia ME, Kieninger M, Ventura ON, Barone V. A reinvestigation of the deceptively simple reaction of toluene with ·OH, and the fate of the benzyl radical. I. A combined thermodynamic and kinetic study on the competition between HO-addition and H-abstraction reactions (unpublished)
Salta Z, Kosmas AM, Segovia ME, Kieninger M, Ventura ON, Barone V. A reinvestigation of the deceptively simple reaction of toluene with ·OH, and the fate of the benzyl radical. II. The “secret” routes to cresols and benzaldehyde (unpublished)
Frisch MJ, Head-Gordon M, Pople JA (1990) Direct MP2 gradient method. Chem Phys Lett 166:275–280
Head-Gordon M, Head-Gordon T (1994) Analytic MP2 frequencies without fifth order storage: theory and application to bifurcated hydrogen bonds in the Water Hexamer. Chem Phys Lett 220:122–128
Coitiño EL, Ventura ON (1993) Isomerization of the formaldehyde radical cation and the failure of MP2. Chem Phys Lett 202:479–482
Soydaş E, Bozkaya U (2015) Assessment of orbital-optimized MP2.5 for thermochemistry and kinetics: dramatic failures of standard perturbation theory approaches for aromatic bond dissociation energies and barrier heights of radical reactions. J Chem Theory Comput 11:1564–1573
Montgomery JA Jr, Frisch MJ, Ochterski JW, Petersson GA (1999) A complete basis set model chemistry VI. Use of density functional geometries and frequencies. J Chem Phys 110:2822–2827
Montgomery JA Jr, Frisch MJ, Ochterski JW, Petersson GA (2000) A complete basis set model chemistry VII. Use of the minimum population localization method. J Chem Phys 112:6532–6542
Becke AD (1993) Density-functional thermochemistry III. The role of exact exchange. J Chem Phys 98:5648–5652
Curtiss LA, Redfern PC, Raghavachari K (2007) Gaussian-4 theory. J Chem Phys 126:084108
Somers KP, Simmie JM (2015) Benchmarking compound methods (CBS-QB3, CBS-APNO, G3, G4, W1BD) against the active thermochemical tables: formation enthalpies of radicals. J Phys Chem A 119:8922–8933
Simmie JM, Somers KP (2015) Benchmarking compound methods (CBS-QB3, CBS-APNO, G3, G4, W1BD) against the active thermochemical tables: a litmus test for cost-effective molecular formation enthalpies. J Phys Chem A 119:7235–7246
Koch W, Holthausen MC (2001) A chemists guide to density functional theory. Wiley, New York
Mardirossian N, Head-Gordon M (2017) Thirty years of density functional theory in computational chemistry: an overview and extensive assessment of 200 density functionals. Mol Phys 115:2315–2372
van Santen JA, DiLabio GA (2015) Dispersion corrections improve the accuracy of both noncovalent and covalent interactions energies predicted by a density-functional theory approximation. J Phys Chem A 119:6703–6713
Jones RO (2015) Density functional theory: its origins, rise to prominence, and future. Revs Mod Phys 87:897–923
Sengupta A, Raghavachari K (2017) Solving the density functional conundrum: elimination of systematic errors to derive accurate reaction enthalpies of complex organic reactions. Org Lett 19:2576–2579
Boese AD (2015) Density functional theory and hydrogen bonds: are we there yet? ChemPhysChem 16:978–985
Yu HS, He X, Li SL, Truhlar DG (2016) MN15: a Kohn–Sham global-hybrid exchange–correlation density functional with broad accuracy for multi-reference and single-reference systems and noncovalent interactions. Chem Sci 7:5032–5051
Lehtola S, Steigemann C, Oliveira MJT, Marques MAL (2018) Recent developments in libxc—a comprehensive library of functionals for density functional theory. SoftwareX 7:1–5
Grimme S, Schreiner PR (2018) Computational chemistry: the fate of current methods and future challenges. Angew Chem Int Ed 57:4170–4176
Gulans A, Kozhevnikov A, Draxl C (2018) Microhartree precision in density functional theory calculations. Phys Rev B 97:161105(R)
Hait D, Head-Gordon M (2018) How accurate is density functional theory at predicting dipole moments? An assessment using a new database of 200 benchmark values. J Chem Theory Comput 14:1969–1981
Irving K, Kieninger M, Ventura ON (2019) Basis Set Effects in the description of the Cl-O bond in ClO and XClO/ClOX isomers (X = H, O and Cl) using DFT and CCSD(T) methods. J Chem. https://doi.org/10.1155/2019/4057848
Petsis G, Salta Z, Kosmas AM, Ventura ON (2019) Theoretical study of the microhydration of 1-chloro and 2-chloro ethanol as a clue for their relative propensity toward dehalogenation. Int J Quantum Chem. https://doi.org/10.1002/qua.25931 (in press)
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Jensen F (2017) Introduction to computational chemistry, 3rd edn. Wiley, New York
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2013) Gaussian 09, Revision D.01. Gaussian Inc., Wallingford
Pople JA, Head-Gordon M, Raghavachari K (1987) Quadratic configuration interaction. A general technique for determining electron correlation energies. J Chem Phys 87:35975
Purvis GD, Bartlett RJ (1982) A full coupled-cluster singles and doubles model: the inclusion of disconnected triples. J Chem Phys 76:1910–1919
Scuseria GE, Schaefer HF III (1989) Is coupled cluster singles and doubles (CCSD) more computationally intensive than quadratic configuration-interaction (QCISD)? J Chem Phys 90:3700–3703
Ruscic B, Pinzon RE, Morton ML, von Laszewski G, Bittner S, Nijsure SG, Amin KA, Minkoff M, Wagner AF (2004) Introduction to active thermochemical tables: several “key” enthalpies of formation revisited. J Phys Chem A 108:9979–9997
Amir-Ebrahimi V, Choplin A, Demaison J, Roussy G (1981) Microwave spectrum of the 13C-ring-monosubstituted toluenes and structure of toluene. J Mol Spectrosc 89:42–52
Noble-Eddy R (2009) Gas-phase electron diffraction studies of unstable molecules. A thesis presented for the degree of Doctor of Philosophy in the College of Science and Engineering at the University of Edinburgh. http://www.era.lib.ed.ac.uk/handle/1842/4101. Web site consulted Dec. 2017
Kortyna A, Samin AJ, Miller TA, Nesbitt DJ (2017) Sub-Doppler infrared spectroscopy of resonance stabilized hydrocarbon intermediates: ν 3/v4 CH stretch modes and CH2 internal rotor dynamics of benzyl radical. Phys Chem Chem Phys 19:29812–29821
Ruscic B, Boggs JE, Burcat A, Csaszar AG, Demaison J, Janoschek R, Martin JML, Morton ML, Rossi MJ, Stanton JF, Szalay PG, Westmoreland PR, Zabel F, Berces T (2005) IUPAC critical evaluation of thermochemical properties of selected radicals. Part I. J Phys Chem Ref Data 34:573–656
Walker JA, Tsang W (1990) Single-pulse shock tube studies on the thermal decomposition of n-butyl phenyl ether, n-pentylbenzene, and phenetole and the heat of formation of phenoxyl and benzyl radicals. J Phys Chem 94:3324–3327
Hippler H, Troe J (1990) Thermodynamic properties of benzyl radicals: enthalpy of formation from toluene, benzyl iodide, and dibenzyl dissociation equilibria. J Phys Chem 94:3803–3806
Tsang W (1996) Heats of formation of organic free radicals by kinetic methods. In: Simões JAM, Greenberg A, Liebman JF (eds) Energetics of organic free radicals. Blackie Academic and Professional, London, pp 22–58
Martinez O Jr, Crabtree KN, Gottlieb CA, Stanton JF, McCarthy MC (2015) An accurate molecular structure of phenyl, the simplest aryl radical. Angew Chem Int Ed 54:1808–1811
Cheng C-W, Lee Y-P, Witek HA (2008) Theoretical investigation of molecular properties of the first excited state of the phenoxyl radical. J Phys Chem A 112:2648–2657
Borisenko KB, Bock ChW, Hargittai I (1996) Molecular geometry of benzaldehyde and salicylaldehyde: a gas-phase electron diffraction and ab initio molecular orbital investigation. J Phys Chem 100:7426–7434
Stevens WR, Ruscic B, Baer T (2010) Heats of formation of C6H5, C6H5 + , and C6H5NO by threshold photoelectron photoion coincidence and active thermochemical tables analysis. J Phys Chem A 114:13134–13145
Sebbar N, Bozzelli JW, Bockhorn H (2011) Thermochemistry and reaction paths in the oxidation reaction of benzoyl radical: c6H5C (=O). J Phys Chem A 115:11897–11914
Simões JAM, Griller D (1989) Enthalpy of formation of the benzoyl radical by photoacoustic calorimetry. Chem Phys Lett 158:175–177
Simões RG, Agapito F, Diogo HP, Minas da Piedade ME (2014) Enthalpy of formation of anisole: implications for the controversy on the O − H bond dissociation enthalpy in phenol. J Phys Chem A 118:11026–11032
Elmaimouni L, Minetti R, Sawerysyn JP, Devolder P (1993) Kinetics and thermochemistry of the reaction of benzyl radical with 02: investigations by discharge flowhaser induced fluorescence between 393 and 433 K. Int J Chem Kinet 25:399–413
Cox JD (1961) The heats of combustion of phenol and the three cresols. Pure Appl Chem 2:125–128
Fattahi A, Kass SR, Liebman JF, Matos MAR, Miranda MS, Morais VMF (2005) The enthalpies of formation of o -, m -, and p -Benzoquinone: gas-phase ion energetics, combustion calorimetry, and quantum chemical computations combined. J Am Chem Soc 127:6116–6122
Sabbah R, Buluku ENLE (1991) Thermodynamic study of three isomers of dihydroxybenzene. Can J Chem 69:481–488
Goton R, Whalley E (1956) Thermodynamic properties of benzoic acid. Can J Chem 34:1506–1507
Papina TS, Pimenova SM, Luk’yanova VA, Kolesov VP (1995) Standard enthalpies of formation of benzyl alcohol and α, α, α-trichlorotoluene. Russ J Phys Chem (Engl Transl) 69:1951–1953
Dorofeeva OV, Ryzhova ON (2016) Enthalpy of formation and O–H bond dissociation enthalpy of phenol: inconsistency between theory and experiment. J Phys Chem A 120:2471–2479
Joback KG, Reid RC (1987) Estimation of pure-component properties from group-contributions. Chem Eng Commun 57:233–243
Cheméo website, see https://www.chemeo.com/cid/64-057-8/Salicyl%20alcohol. Visited 23rd Dec 2017
da Silva G, Hamdan MR, Bozzelli JW (2009) Oxidation of the benzyl radical: mechanism, thermochemistry, and kinetics for the reactions of benzyl hydroperoxide. J Chem Theor Comput 5:3185–3194
da Silva G, Bozzelli JW (2009) Kinetic modeling of the benzyl + HO2 reaction. Proc. Combust. Instit. 32:287–294
Fenter FF, Noziere B, Caralp F, Lesclaux R (1994) Study of the kinetics and equilibrium of the benzyl-radical association reaction with molecular oxygen. Int J Chem Kinet 26:171–189
Acknowledgements
PEDECIBA (Uy), CSIC (UdelaR, Uy) and ANII (Uy) are gratefully acknowledged for sustained funding of the theoretical atmospheric chemistry program in which the toluene project is included. Some of the calculations reported in this paper were performed in ClusterUY, a newly installed platform for high-performance scientific computing at the National Supercomputing Center, Uruguay.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published as part of the special collection of articles derived from the 11th Congress on Electronic Structure: Principles and Applications (ESPA-2018).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ventura, O.N., Kieninger, M., Salta, Z. et al. Enthalpies of formation of the benzyloxyl, benzylperoxyl, hydroxyphenyl radicals and related species on the potential energy surface for the reaction of toluene with the hydroxyl radical. Theor Chem Acc 138, 115 (2019). https://doi.org/10.1007/s00214-019-2500-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-019-2500-8