Abstract
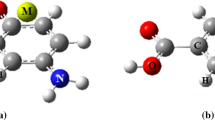
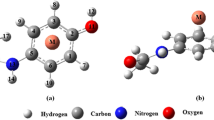
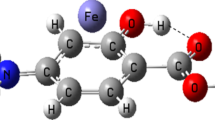
The molecular modeling analysis via density functional theory (DFT) calculations is performed on all the formed complexes from interaction between mono- and divalent metal cations (Li+, Na+, K+, Be2+, Mg2+ and Ca2+) with acetaminophen. The interaction energies are calculated by M06-2X method in the gas phase and the solution. The obtained structures are analyzed by topological parameters in terms of electron density (ρ) and its Laplacian (∇2ρ) at the bond critical point (BCP) using the atoms in molecules (AIM) methodology. The evaluated results from calculations suggest that the strongest interaction and the highest electron density at BCP are related to the Be2+ complex. In addition, the natural bond orbital (NBO) analysis is performed to investigate the charge distribution in the related complexes. The MEP (molecular electrostatic potential) is given the visual representation of the chemically active sites and comparative reactivity of atoms. Finally, the quantum molecular descriptors such as energy gap, electronic chemical potential, global hardness and electrophilicity index are calculated to evaluate the electronic properties, stability and reactivity of the analyzed complexes.






Similar content being viewed by others
References
Dinis TC, Maderia VM, Almeida LM (1994) Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 315:161–169
Shertzer HG, Schneider SN, Kendig EL, Clegg DJ, D’alessio DA, Genter MB (2008) Acetaminophen normalizes glucose homeostasis in mouse models for diabetes. Biochem Pharmacol 75:1402–1410
Blough ER, Wu M (2011) Acetaminophen: beyond pain and fever-relieving. Front Pharmacol 2:72-1–72-6
Jóźwiak-Bebenista M, Nowak JZ (2014) Paracetamol: mechanism of action, applications and safety concern. Acta Pol Pharm 71:11–23
Alves C, Borges R, Da Silva A (2006) Density functional theory study of metabolic derivatives of the oxidation of paracetamol. Int J Quantum Chem 106:2617–2623
Ghosh J, Das J, Manna P, Sil PC (2010) Acetaminophen induced renal injury via oxidative stress and TNF-alpha production: therapeutic potential of arjunolic acid. Toxicology 268:8–18
Agarwal R, Macmillan-Crow LA, Rafferty TM, Saba H, Roberts DW, Fifer EK, James LP, Hinson JA (2011) Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J Pharmacol Exp Ther 337:110–118
Thomas SH (1993) Paracetamol (acetaminophen) poisoning. Pharmacol Ther 60:91–120
Sanghavi BJ, Srivastava AK (2010) Simultaneous voltammetric determination of acetaminophen, aspirin and caffeine using an in situ surfactant-modified multiwalled carbon nanotube paste electrode. Electrochim Acta 55:8638–8648
Yoosefian M, Ansarinik Z, Etminan N (2016) Density functional theory computational study on solvent effect, molecular conformations, energies and intramolecular hydrogen bond strength in different possible nano-conformers of acetaminophen. J Mol Liq 213:115–121
Schneider HJ (2009) Binding mechanisms in supramolecular complexes. Angew Chem Int Ed 48:3924–3977
Ma JC, Dougherty DA (1997) The cation–π interaction. Chem Rev 97(5):1303–1324
Rimola A, Rodríguez-Santiago L, Sodupe M (2006) Cation–π interactions and oxidative effects on Cu+ and Cu2+ binding to Phe, Tyr, Trp, and His amino acids in the gas phase. insights from first-principles calculations. J Phys Chem B 110:24189–24199
Fukin GK, Lindeman SV, Kochi JK (2002) Molecular structures of cation…pi(arene) interactions for alkali metals with pi- and sigma-modalities. J Am Chem Soc 124:8329–8336
Ebrahimi A, Habibi Khorassani M, Masoodi HR (2011) Cation–π versus anion–π interactions: a theoretical NMR study. Chem Phys Lett 504:118–124
Kim CK, Zhang H, Yoon SH, Won J, Lee MJ, Kim CK (2009) Effects of basis set superposition error on optimized geometries and complexation energies of organo-alkali metal cation complexes. J Phys Chem A 113:513–519
Mo Y, Subramanian G, Gao J, Ferguson DM (2002) Cation-pi interactions: an energy decomposition analysis and its implication in delta-opioid receptor-ligand binding. J Am Chem Soc 124:4832–4837
Srinivasa Rao J, Zipse H, Narahari Sastry G (2009) Explicit solvent effect on cation–π interactions: a first principle investigation. J Phys Chem B 113:7225–7236
Rute Neves A, Alexandrino Fernandes P, João Ramos M (2011) The accuracy of density functional theory in the description of cation–π and π–hydrogen bond interactions. J Chem Theory Comput 7:2059–2067
Crowley PB, Golovin A (2005) Cation–π interactions in protein–protein interfaces. Proteins 59:231–239
Cubero E, Luque FJ, Orozco M (1998) Is polarization important in cation–π interactions? Proc Natl Acad Sci 95:5976–5980
Vyas N, Ojha AK (2011) A study on interaction of Be++, Mg++ and Ca++ with phenylalanine: binding energies, metal ion affinities and IR signature of complex stability. Vib Spectrosc 56:42–50
Ragsdale SW (2006) Metals and their scaffolds to promote difficult enzymatic reactions. Chem Rev 106:3317–3337
Liu J, Xia X, Li Y, Wang H, Li Z (2013) Theoretical study on the interaction of glutathione with group IA (Li+, Na+, K+), IIA (Be2+, Mg2+, Ca2+), and IIIA (Al3+) metal cations. Struct Chem 24:251–261
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03 (Revision A.7) Gaussian, Inc., Pittsburgh
Tomasi J, Cammi R, Mennucci B, Cappelli C, Corni S (2002) Molecular properties in solution described with a continuum solvation model. Phys Chem Chem Phys 4:5697–5712
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, New York
Biegler-König FW, Bader RFW, Tang TH (1982) Calculation of the average properties of atoms in molecules. II. J Comput Chem 3:317–328
Biegler-König FW, Schonbohm J, Derdan R, Bayles D, Bader R (2000) AIM2000, Version 2.000
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor–acceptor viewpoint. Chem Rev 88:899–926
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1992) NBO, Version 3.1
Hokmabady L, Raissi H, Khanmohammadi A (2016) Interactions of the 5-fluorouracil anticancer drug with DNA pyrimidine bases: a detailed computational approach. Struct Chem 27:487–504
Chattaraj PK, Poddar A (1999) Molecular reactivity in the ground and excited electronic states through density-dependent local and global reactivity parameters. J Phys Chem A 103:8691–8699
Pearson RG (1997) Chemical hardness—applications from molecules to solids. VCH-Wiley, Weinheim
Parr RG, Lv Szentpály, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924
Sen KD, Jorgensen CK (1987) Electronegativity, structure and bonding. Springer, New York
Koopmans T (1933) Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den einzelnen Elektronen eines Atoms. Physica 1:104–113
Garau C, Frontera A, Quiñonero D, Ballester P, Costa A, Deyá PM (2004) Cation–π versus anion–π interactions: a comparative ab initio study based on energetic, electron charge density and aromatic features. Chem Phys Lett 392:85–89
Garura C, Frontera A, Quiñonero D, Ballester P, Costa A, Deyá PM (2003) A topological analysis of the electron density in anion-π interactions. Chem Phys Chem 4:1344–1348
Weinhold F, Schleyer PVR (eds) (1998) Encyclopedia of computational chemistry. Wiley, New York
Roohi H, Noruozi AR, Salemi S, Sharaki J (2008) Theoretical study of solvent effects on the conformational preference in CH2FWH (W=O, S) using PCM and IPCM methods. J Mol Liq 143:119–124
Raissi H, Yoosefian M (2012) Substituent effect on the reaction mechanism of proton transfer in formamide. Int J Quantum Chem 112:2378–2381
Raissi H, Yoosefian M, Hajizadeh A, Karimi M, Farzad F (2012) Theoretical description of substituent effects in 2,4-pentanedione: AIM, NBO, and NMR study. Bull Chem Soc Jpn 85:87–92
Domingo LR, Ríos-Gutiérrez M, Pérez P (2016) Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 21:748(1–22)
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron 5:4417–4423
Pascual-ahuir JL, Silla E, Tuñon I (1994) GEPOL: an improved description of molecular surfaces. III. A new algorithm for the computation of a solvent-excluding surface. J Comput Chem 15:1127–1138
Cossi M, Mennucci B, Cammi R (1996) Analytical first derivatives of molecular surfaces with respect to nuclear coordinates. J Comput Chem 17:57–73
Cossi M, Barone V, Cammi R, Tomasi J (1996) Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem Phys Lett 255:327–335
Thanikachalam V, Periyanayagasamy V, Jayabharathi J, Manikandan G, Saleem H, Subashchandrabose S, Erdogdu Y (2012) FT-Raman, FT-IR spectral and DFT studies on (E)-1-4-nitrobenzylidenethiocarbonohydrazide. Spectrochim Acta A Mol Biomol Spectrosc 87:86–95
Sridevi C, Shanthi G, Velraj G (2012) Structural, vibrational, electronic, NMR and reactivity analyses of 2-amino-4H-chromene-3-carbonitrile (ACC) by ab initio HF and DFT calculations. Spectrochim Acta A Mol Biomol Spectrosc 89:46–54
Raissi H, Khanmohammadi A, Mollania F (2013) A theoretical DFT study on the structural parameters and intramolecular hydrogen-bond strength in substituted (Z)-N-(thionitrosomethylene) thiohydroxylamine systems. Bull Chem Soc Jpn 86:1261–1271
Onsager L (1936) Electric moments of molecules in liquids. J Am Chem Soc 58:1486–1493
Kirkwood JG (1935) Statistical mechanics of fluid mixtures. J Chem Phys 3:300–313
Acknowledgements
The support of this work by Vali-e-Asr University of Rafsanjan is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mohammadi, M., Khanmohammadi, A. Molecular structure, QTAIM and bonding character of cation–π interactions of mono- and divalent metal cations (Li+, Na+, K+, Be2+, Mg2+ and Ca2+) with drug of acetaminophen. Theor Chem Acc 138, 101 (2019). https://doi.org/10.1007/s00214-019-2492-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-019-2492-4