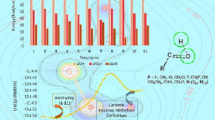
Abstract
Understanding carbocation formation is a central concern for all chemical sciences. The widely accepted explanation in terms of inductive/field and delocalization effects is based on quantities that are not straightforwardly computed in popular electronic structure methods. This work reports an alternative approach to the carbocation formation problem based on energy decomposition analysis, more specifically, CMOEDA. The order of stability for carbocations formation was successfully accounted in terms of the energy components. The focus of the analysis shifts from the product of the reaction, i.e., the carbocation itself, to the reactant neutral molecule. Notably, exchange repulsions are the largest energy contribution to increase carbocation stability in the order methyl, primary, secondary and tertiary. Polarization (orbital relaxation) plays a secondary role. Insertion of bulky groups increases the repulsion with the incipient anion (a hydride ion) and decreases the strength of the C–H bond. This pattern is confirmed for several other hydrocarbon cases. Additional systems like halomethanes, amino- and nitro-derivatives are also described.










Similar content being viewed by others
References
Aue DH (2011) Wiley Interdiscip Rev Comput Mol Sci 1:487–508
Naredla RR, Klumpp DA (2013) Chem Rev 113:6905–6948
Olah GA (2001) J Org Chem 66:5944–5957
Alamiddine A, Humbel S (2014) Front Chem 1:1–9
Jalife S, Martínes-Guajardo G, Zavala-Oseguera C, Fernández-Herrera M, Schleyer P, Merino G (2014) Eur J Org Chem 7955–7959
Sandbeck DJS, Markewich DJ, East ALL (2016) J Org Chem 81:1410–1415
Moss R (2014) J Phys Org Chem 27:374–379
Chiavarino B, Crestoni ME, Fornarini S, Lemaire J, Aleese LM, Maître P (2004) ChemPhysChem 5:1679–1685
Robbins AM, Jin P, Brinck T, Murray JS, Politzer P (2006) Int J Quantum Chem 106:2904–2909
Morokuma K (1971) J Chem Phys 55:1236–1244
Lynch K, Maloney A, Sowell A, Wang C, Mo Y, Karty JM (2015) Phys Chem Chem Phys 17:138–144
Su P, Li H (2009) J Chem Phys 131:014102-1–014102-15
te Velde G, Bickelhaupt FM, Baerends EJ, Fonseca Guerra C, van Gisbergen SJA, Snijders JG, Ziegler T (2001) J Comput Chem 22:931–967
Stasyuk OA, Szatylowicz H, Krygowski TM, Fonseca Guerra C (2016) Phys Chem Chem Phys 18:11624–11633
Dancini-Pontes I, Fernandes-Machado N, Souza M, Pontes RM (2015) App Catal A 491:86–93
Banu T, Ghosh D, Debnath T, Sen K, Das AK (2015) RSC Adv 5:57647–57656
Baranac-Stojanović M (2015) Struct Chem 26:989–996
Aleksić J, Stojanović M, Baranac-Stajanović M (2015) J Org Chem 80:10197–10207
Karir G, Fatima M, Viswanathan KS (2016) J Chem Sci 128:1557–1569
Thellamurege N, Hirao H (2013) Molecules 18:6782–6791
Pontes RM, Basso EA, Martins DE, Madeira RM (2017) J Phys Chem A 121:4993–5004
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Kiprof P, Miller SR, Frank MA (2006) J Mol Struct (THEOCHEM) 764:61–67
Pople JA (1987) Chem Phys Lett 137:10–12
Curtiss LA, Pople JA (1998) J Chem Phys 88:7405–7409
Liang C, Hamilton TP, Schaefer HF (1990) J Chem Phys 92:3653–3658
Psciuk BT, Bendererskii VA, Schlegel HB (2007) Theor Chem Acc 118:75–80
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pontes, R.M. Theoretical perspectives on carbocation chemistry from energy decomposition analysis. Theor Chem Acc 137, 56 (2018). https://doi.org/10.1007/s00214-018-2232-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-018-2232-1