Abstract
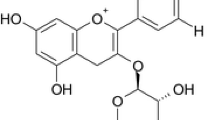
A multi-level computational protocol is devised to calculate the absorption spectra in ethanol solution of a series of anthocyanidins relevant for dye-sensitized solar cells. The protocol exploits the high accuracy of second-order multi-reference perturbation theory to correct the results of the more feasible TD-DFT calculations, which were performed on hundreds of configurations sampled from molecular dynamics (MD) trajectories. The latter were purposely carried out with accurate and reliable force fields, specifically parameterized against quantum mechanical data, for each of the investigated dyes. Besides yielding maximum absorption wavelengths very close to the experimental values, the present approach was also capable of predicting reliable band shapes, even accounting for the subtle differences observed along the homolog series. Finally, the atomistic description achieved by MD simulations allowed for a deep insight into the different micro-solvation patterns around each anthocyanidin and their effects on the resulting dye’s properties. This work can be considered as a step toward the implementation of a computational protocol able to simulate the whole system formed by the organic dye and its heterogeneous embedding that constitutes dye-sensitized solar cells.











Similar content being viewed by others
References
O’Regan B, Grätzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737
Monat JE, Rodriguez JH, McCusker JK (2002) Ground- and Excited-State Electronic Structures of the Solar Cell Sensitizer Bis(4,4′-dicarboxylato-2,2′-bipyridine)bis(isothiocyanato)ruthenium(II). J Phys Chem A 106:7399
De Angelis F, Fantacci S, Selloni A, Grätzel M, Nazeeruddin MK (2007) Influence of the sensitizer adsorption mode on the open-circuit potential of dye-sensitized solar cells. Nano Lett 7:3189–3195
Graetzel M (2009) Recent advances in sensitized mesoscopic solar cells. Acc Chem Res 42:1788–1798
De Angelis F, Fantacci S, Selloni A, Nazeeruddin MK, Grätzel M (2011) First-principles modeling of the adsorption geometry and electronic structure of Ru(II) Dyes on extended TiO2 substrates for dye-sensitized solar cell applications. J Phys Chem C 114:6054–6061
Bignozzi CA, Argazzi R, Boaretto R, Busatto E, Carli S, Ronconi F, Caramori S (2013) The role of transition metal complexes in dye sensitized solar devices. Coord Chem Rev 257:1472–1492
Kuang D, Comte P, Zakeeruddin SM, DlP Hagberg, Karlsson KM, Sun L, Nazeeruddin MK, Grätzel M (2011) Stable dye-sensitized solar cells based on organic chromophores and ionic liquid electrolyte. Sol Energy 85:1189
Odobel F, Le Pleux L, Pellegrin Y, Blart E (2010) New photovoltaic devices based on the sensitization of p-type semiconductors: challenges and opportunities. Acc Chem Res 43:1063–1071
Pastore M, Fantacci S, De Angelis F (2010) Ab initio determination of ground and excited state oxidation potentials of organic chromophores for dye-sensitized solar cells. J Phys Chem C 114:22742
Plannels M, Pellejà L, Clifford JN, Pastore M, De Angelis F, López N, Marder S, Palomares E (2011) Energy levels, charge injection, charge recombination and dye regeneration dynamics for donor–acceptor π-conjugated organic dyes in mesoscopic TiO2 sensitized solar cells. Energy Environ Sci 4:1820
Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H (2010) Dye-sensitized solar cells. Chem Rev 110:6595
Li L-L, Diau EW-G (2013) Porphyrin-sensitized solar cells. Chem Soc Rev 42:291–304
Jinchu I, Sreekala CO, Sreelatha KS (2014) Dye sensitized solar cell using natural dyes as chromophores—review. In: Pandikumar A, Jothilakshmi R (eds) Materials science forum, vol 771. Trans Tech Publications Ltd, Laublsrutistr 24, Ch-8717 Stafa-Zurich, Switzerland, pp 39–51. doi:10.4028/www.scientific.net/MSF.771.39
Narayan MR (2012) Review: dye sensitized solar cells based on natural photosensitizers. Renev Sust Energ Rev 16:208–215
Senthil TS, Muthukumarasamy N, Velauthapillai D, Agilan S, Thambidurai M, Balasundaraprabhu R (2011) Natural dye (cyanidin 3-o-glucoside) sensitized nanocrystalline TiO2 solar cell fabricated using liquid electrolyte/quasi-solid-state polymer electrolyte. Renew Energ 36:2484–2488
Castañeda-Ovando A, Pacheco-Hernández MdL, Páez-Hernández ME, Rodríguez JA, Galán-Vidal CA (2009) Chemical studies of anthocyanins: a review. Food Chem 113:859–871
Falcone Ferreyra ML, Rius SP, Casati P (2012) Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci 3:222
Halbwirth H (2010) The creation and physiological relevance of divergent hydroxylation patterns in the flavonoid pathway. Int J Mol Sci 11:595–621
Rustioni L, Di Meo F, Guillaume M, Failla O, Trouillas P (2013) Tuning color variation in grape anthocyanins at the molecular scale. Food Chem 141:4349–4357
Trouillas P, Di Meo F, Gierschner J, Linares M, Sancho-García JC, Otyepka M (2015) Optical properties of wine pigments: theoretical guidelines with new methodological perspectives. Tetrahedron 71:3079–3088
Shahid M, Shahid ul I, Mohammad F (2013) Recent advancements in natural dye applications: a review. J Clean Prod 53:310–331
Calogero G, Marco GD (2008) Red Sicilian orange and purple eggplant fruits as natural sensitizers for dye-sensitized solar cells. Sol Energy Mater Sol Cells 92:1341–1346
Gómez-Ortíz NM, Vázquez-Maldonado IA, Pérez-Espadas AR, Mena-Rejón GJ, Azamar-Barrios JA, Oskam G (2010) Dye-sensitized solar cells with natural dyes extracted from achiote seeds. Sol Energy Mater Sol Cells 94:40–44
Ekanayake P, Kooh MRR, Kumara NTRN, Lim A, Petra MI, Voo NY, Lim CM (2013) Combined experimental and DFT–TDDFT study of photo-active constituents of Canarium odontophyllum for DSSC application. Chem Phys Lett 585:121–127
Ludin NA, Mahmoud AMA-A, Mohamad AB, Kadhum AAH, Sopian K, Karim NSA (2014) Review on the development of natural dye photosensitizer for dye-sensitized solar cells. Renev Sust Energ Rev 31:386–396
Goto T, Kondo T (1991) Struktur und molekulare Stapelung von Anthocyanen—Variation der Blutenfarben. Angew Chem 103:17–33
Di Meo F, Sancho Garcia JC, Dangles O, Trouillas P (2012) Highlights on anthocyanin pigmentation and copigmentation: a matter of flavonoid π-stacking complexation to be described by DFT-D. J Chem Theor Comput 8:2034–2043
Brouillard R, Mazza G, Saad Z, Albrecht-Gary AM, Cheminat A (1989) The co-pigmentation reaction of anthocyanins: a microprobe for the structural study of aqueous solutions. J Am Chem Soc 111:2604–2610
Harborne JB (1958) Spectral methods of characterizing anthocyanins. Biochem J 70:22–28
Dai Q, Rabani J (2002) Photosensitization of nanocrystalline TiO2 films by anthocyanin dyes. J Photochern Photobiol A 148:17–24
Liu Z (2008) Theoretical studies of natural pigments relevant to dye-sensitized solar cells. J Mol Struct THEOCHEM 862:44–48
Calzolari A, Varsano D, Ruini A, Catellani A, Tel-Vered R, Yildiz HB, Ovits O, Willner I (2009) Optoelectronic properties of natural cyanin dyes. J Phys Chem A 113:8801–8810
Ge X, Calzolari A, Baroni S (2015) Optical properties of anthocyanins in the gas phase. Chem Phys Lett 618:24–29
Anouar EH, Gierschner J, Duroux J-L, Trouillas P (2012) UV/Visible spectra of natural polyphenols: a time-dependent density functional theory study. Food Chem 131:79–89
Ge X, Timrov I, Binnie S, Biancardi A, Calzolari A, Baroni S (2015) Accurate and inexpensive prediction of the color optical properties of anthocyanins in solution. J Phys Chem A 119:3816–3822
Soto-Rojo R, Baldenebro-López J, Flores-Holguín N, Glossman-Mitnik D (2014) Comparison of several protocols for the computational prediction of the maximum absorption wavelength of chrysanthemin. J Mol Model 20:2378
Millot M, Di Meo F, Tomasi S, Boustie J, Trouillas P (2012) Photoprotective capacities of lichen metabolites: a joint theoretical and experimental study. J Photochem Photobiol B 111:17–26
Malcıoğlu OB, Calzolari A, Gebauer R, Varsano D, Baroni S (2011) Dielectric and thermal effects on the optical properties of natural dyes: a case study on solvated cyanin. J Am Chem Soc 133:15425–15433
Sakata K, Saito N, Honda T (2006) Ab initio study of molecular structures and excited states in anthocyanidins. Tetrahedron 62:3721–3731
Barone V, Ferretti A, Pino I (2012) Absorption spectra of natural pigments as sensitizers in solar cells by TD-DFT and MRPT2: protonated cyanidin. Phys Chem Chem Phys 14(46):16130–16137
Avila Ferrer FJ, Cerezo J, Stendardo E, Improta R, Santoro F (2013) Insights for an accurate comparison of computational data to experimental absorption and emission spectra: beyond the vertical transition approximation. J Chem Theory Comput 9:2072–2082
Muniz-Miranda F, Pedone A, Battistelli G, Montalti M, Bloino J, Barone V (2015) Benchmarking TD-DFT against vibrationally resolved absorption spectra at room temperature: 7-aminocoumarins as test cases. J Chem Theory Comput 11:5371–5384
Charaf-Eddin A, Cauchy T, Fc-X Felpin, Jacquemin D (2014) Vibronic spectra of organic electronic chromophores. RSC Adv 4:55466–55472
Lopez GV, Chang C-H, Johnson PM, Hall GE, Sears TJ, Markiewicz B, Milan M, Teslja A (2012) What is the best DFT functional for vibronic calculations? A comparison of the calculated vibronic structure of the S 1-S 0 transition of phenylacetylene with cavity ringdown band intensities. J Phys Chem A 116:6750–6758
Jacquemin D, Brémond E, Al Planchat, Ciofini I, Adamo C (2011) TD-DFT vibronic couplings in anthraquinones: from basis set and functional benchmarks to applications for industrial dyes. J Chem Theory Comput 7:1882–1892
Dierksen M, Grimme S (2004) The vibronic structure of electronic absorption spectra of large molecules: a time-dependent density functional study on the influence of “Exact” Hartree–Fock exchange. J Phys Chem A 108:10225–10237
Cacelli I, Ferretti A, Prampolini G (2014) Perturbative multireference configuration interaction (CI-MRPT2) calculations in a focused dynamical approach: a computational study of solvatochromism in pyrimidine. J Phys Chem A 119(21):5250–5259
De Mitri N, Monti S, Prampolini G, Barone V (2013) Absorption and emission spectra of a flexible dye in solution: a computational time-dependent approach. J Chem Theory Comput 9:4507–4516
De Mitri N, Prampolini G, Monti S, Barone V (2014) Structural, dynamic and photophysical properties of a fluorescent dye incorporated in an amorphous hydrophobic polymer bundle. Phys Chem Chem Phys 16:16573–16587
Pedone A, Prampolini G, Monti S, Barone V (2011) Realistic modeling of fluorescent dye-doped silica nanoparticles: a step toward the understanding of their enhanced photophysical properties. Chem Mater 23:5016–5023
Marenich AV, Cramer CJ, Truhlar DG (2015) Electronic absorption spectra and solvatochromic shifts by the vertical excitation model: solvated clusters and molecular dynamics sampling. J Phys Chem B 119:958–967
Cerezo J, Prampolini G, Santoro F (2015) Comparing classical approaches with empirical or quantum-mechanically derived force-fields for the simulations of electronic lineshapes: application to coumarin dyes. Theor Chem Acc 135(5):1–21
Cacelli I, Prampolini G (2007) Parametrization and validation of intramolecular force fields derived from DFT calculations. J Chem Theor Comput 3:1803–1817
Barone V, Cacelli I, De Mitri N, Licari D, Monti S, Prampolini G (2013) JOYCE and ULYSSES: integrated and user-friendly tools for the parameterization of intramolecular force fields from quantum mechanical data. Phys Chem Chem Phys 15:3736–3751
Cacelli I, Ferretti A, Prampolini G (2015) Perturbative multireference configuration interaction (CI-MRPT2) calculations in a focused dynamical approach: a computational study of solvatochromism in pyrimidine. J Phys Chem A 119:5250–5259
Cacelli I, Ferretti A, Prampolini G, Barone V (2015) BALOO: a fast and versatile code for accurate multireference variational/perturbative calculations. J Chem Theor Comput 11:2024–2035
AlD Laurent, Jacquemin D (2013) TD-DFT benchmarks: a review. Int J Quantum Chem 113:2019–2039
Jacquemin D, Bahers TL, Adamo C, Ciofini I (2012) What is the best atomic charge model to describe through-space charge-transfer excitations? Phys Chem Chem Phys 14:5383–5388
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3093
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09. Gaussian Inc, Wallingford
Gordon MS, Schmidt MW (2005) GAMESS. Theory and Applications of Computational Chemistry: the first forty years. Elsevier, Amsterdam
Cimiraglia R (1984) Second order perturbation correction to ci energies by use of diagrammatic techniques: an improvement to the Cipsi algorithm. J Chem Phys 83:174
Jorgensen WL, Maxwell DS, Tirado-rives J (1996) Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J Am Chem Soc 7863:11225–11236
Marenich AV, Jerome SV, Cramer CJ, Truhlar DG (2012) Charge Model 5: an extension of hirshfeld population analysis for the accurate description of molecular interactions in gaseous and condensed phases. J Chem Theory Comput 8:527–541
Aaqvist J (1990) Ion-water interaction potentials derived from free energy perturbation simulations. J Phys Chem 94:8021–8024
Caleman C, van Maaren PJ, Hong M, Hub JS, Costa LT, van der Spoel D (2012) Force field benchmark of organic liquids: density, enthalpy of vaporization, heat capacities, surface tension, isothermal compressibility, volumetric expansion coefficient, and dielectric constant. J Chem Theory Comput 8:61–74
Hess B, Kutzner C, van der Spoel D, Lindahl E (2008) GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4(3):435–447
Bussi G, Donadio D, Parrinello M (2007) Canonical sampling through velocity rescaling. J Chem Phys 126:014101
Parrinello M (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52:7182
Acknowledgments
The research leading to these results has received funding from the Italian Ministry of Instruction, University and Research (MIUR), through PRIN 2010–11, 2010PFLRJR (PROxi) and 2010FM738P. Dr. Javier Cerezo is gratefully acknowledged by GP for the many useful discussions.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published as part of the special collection of articles “Health & Energy from the Sun”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cacelli, I., Ferretti, A. & Prampolini, G. Predicting light absorption properties of anthocyanidins in solution: a multi-level computational approach. Theor Chem Acc 135, 156 (2016). https://doi.org/10.1007/s00214-016-1911-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-1911-z