Abstract
Rationale
Accumulating evidence implicates the nicotinic cholinergic system in autism spectrum disorder (ASD) pathobiology. Neuropathologic studies suggest that nicotinic acetylcholine (ACh) receptor (nAChR) subtypes are altered in brain of autistic individuals. In addition, strategies that increase ACh, the neurotransmitter for nicotinic and muscarinic receptors, appear to improve cognitive deficits in neuropsychiatric disorders and ASD.
Objective
The aim of this study is to examine the role of the nicotinic cholinergic system on social and repetitive behavior abnormalities and exploratory physical activity in a well-studied model of autism, the BTBR T+ Itpr3 tf/J (BTBR) mouse.
Methods
Using a protocol known to up-regulate expression of brain nAChR subtypes, we measured behavior outcomes before and after BTBR and C57BL/6J (B6) mice were treated (4 weeks) with vehicle or nicotine (50, 100, 200, or 400 μg/ml).
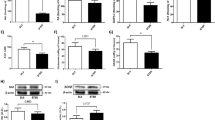
Results
Increasing nicotine doses were associated with decreases in water intake, increases in plasma cotinine levels, and at the higher dose (400 μg/ml) with weight loss in BTBR mice. At lower (50, 100 μg/ml) but not higher (200, 400 μg/ml) doses, nicotine increased social interactions in BTBR and B6 mice and at higher, but not lower doses, it decreased repetitive behavior in BTBR. In the open-field test, nicotine at 200 and 400 μg/ml, but not 100 μg/ml compared with vehicle, decreased overall physical activity in BTBR mice.
Conclusions
These findings support the hypotheses that the nicotinic cholinergic system modulates social and repetitive behaviors and may be a therapeutic target to treat behavior deficits in ASD. Further, the BTBR mouse may be valuable for investigations of the role of nAChRs in social deficits and repetitive behavior.







Similar content being viewed by others
References
Alkondon M, Pereira EF, Almeida LE, Randall WR, Albuquerque EX (2000) Nicotine at concentrations found in cigarette smokers activates and desensitizes nicotinic acetylcholine receptors in CA1 interneurons of rat hippocampus. Neuropharmacology 39:2726–2739
Alkondon M, Pereira EF, Yu P, Arruda EZ, Almeida LE, Guidetti P, Fawcett WP, Sapko MT, Randall WR, Schwarcz R, Tagle DA, Albuquerque EX (2004) Targeted deletion of the kynurenine aminotransferase ii gene reveals a critical role of endogenous kynurenic acid in the regulation of synaptic transmission via alpha7 nicotinic receptors in the hippocampus. J Neurosci 24:4635–4648
Almeida LE, Pereira EF, Alkondon M, Fawcett WP, Randall WR, Albuquerque EX (2000) The opioid antagonist naltrexone inhibits activity and alters expression of alpha7 and alpha4beta2 nicotinic receptors in hippocampal neurons: implications for smoking cessation programs. Neuropharmacology 39:2740–2755
Almeida LE, Pereira EF, Camara AL, Maelicke A, Albuquerque EX (2004) Sensitivity of neuronal nicotinic acetylcholine receptors to the opiate antagonists naltrexone and naloxone: receptor blockade and up-regulation. Bioorg Med Chem Lett 14:1879–1887
Arnold LE, Aman MG, Hollway J, Hurt E, Bates B, Li X, Farmer C, Anand R, Thompson S, Ramadan Y, Williams C (2012) Placebo-controlled pilot trial of mecamylamine for treatment of autism spectrum disorders. J Child Adolesc Psychopharmacol 22:198–205
Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, Deckersbach T, Kelly JF, Freudenreich O, Goff DC, Evins AE (2008) The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology 33:480–490
Bencherif M, Fowler K, Lukas RJ, Lippiello PM (1995) Mechanisms of up-regulation of neuronal nicotinic acetylcholine receptors in clonal cell lines and primary cultures of fetal rat brain. J Pharmacol Exp Ther 275:987–994
Buckley AW, Sassower K, Rodriguez AJ, Jennison K, Wingert K, Buckley J, Thurm A, Sato S, Swedo S (2011) An open label trial of donepezil for enhancement of rapid eye movement sleep in young children with autism spectrum disorders. J Child Adolesc Psychopharmacol 21:353–357
Clayton JA, Collins FS (2014) Policy: NIH to balance sex in cell and animal studies. Nature 509:282–283
Falasca S, Ranc V, Petruzziello F, Khani A, Kretz R, Zhang X, Rainer G (2014) Altered neurochemical levels in the rat brain following chronic nicotine treatment. J Chem Neuroanat 59–60:29–35
Ghaleiha A, Ghyasvand M, Mohammadi MR, Farokhnia M, Yadegari N, Tabrizi M, Hajiaghaee R, Yekehtaz H, Akhondzadeh S (2013) Galantamine efficacy and tolerability as an augmentative therapy in autistic children: a randomized, double-blind, placebo-controlled trial. J Psychopharmacol 28:677–685
Gold M, Newhouse PA, Howard D, Kryscio RJ (2012) Nicotine treatment of mild cognitive impairment: a 6-month double-blind pilot clinical trial. Neurology 78:1895, author reply 1895
Gotti C, Riganti L, Vailati S, Clementi F (2006) Brain neuronal nicotinic receptors as new targets for drug discovery. Curr Pharm Des 12:407–428
Grabus SD, Martin BR, Batman AM, Tyndale RF, Sellers E, Damaj MI (2005) Nicotine physical dependence and tolerance in the mouse following chronic oral administration. Psychopharmacology 178:183–192
Han S, Tai C, Jones CJ, Scheuer T, Catterall WA (2014) Enhancement of inhibitory neurotransmission by GABAA receptors having alpha2,3-subunits ameliorates behavioral deficits in a mouse model of autism. Neuron 81:1282–1289
Heishman SJ, Kleykamp BA, Singleton EG (2010) Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology 210:453–469
Jensen AA, Frolund B, Liljefors T, Krogsgaard-Larsen P (2005) Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J Med Chem 48:4705–4745
Karvat G, Kimchi T (2014) Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacology 39:831–840
Lam KS, Aman MG, Arnold LE (2006) Neurochemical correlates of autistic disorder: a review of the literature. Res Dev Disabil 27:254–289
Lambe EK, Picciotto MR, Aghajanian GK (2003) Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology 28:216–225
Landis SC, Amara SG, Asadullah K, Austin CP, Blumenstein R, Bradley EW, Crystal RG, Darnell RB, Ferrante RJ, Fillit H, Finkelstein R, Fisher M, Gendelman HE, Golub RM, Goudreau JL, Gross RA, Gubitz AK, Hesterlee SE, Howells DW, Huguenard J, Kelner K, Koroshetz W, Krainc D, Lazic SE, Levine MS, Macleod MR, McCall JM, Moxley RT 3rd, Narasimhan K, Noble LJ, Perrin S, Porter JD, Steward O, Unger E, Utz U, Silberberg SD (2012) A call for transparent reporting to optimize the predictive value of preclinical research. Nature 490:187–191
Lee M, Martin-Ruiz C, Graham A, Court J, Jaros E, Perry R, Iversen P, Bauman M, Perry E (2002) Nicotinic receptor abnormalities in the cerebellar cortex in autism. Brain 125:1483–1495
Levin ED (2013) Complex relationships of nicotinic receptor actions and cognitive functions. Biochem Pharmacol 86:1145–1152
Levin ED, Conners CK, Silva D, Canu W, March J (2001) Effects of chronic nicotine and methylphenidate in adults with attention deficit/hyperactivity disorder. Exp Clin Psychopharmacol 9:83–90
Lewejohann L, Reinhard C, Schrewe A, Brandewiede J, Haemisch A, Gortz N, Schachner M, Sachser N (2006) Environmental bias? Effects of housing conditions, laboratory environment and experimenter on behavioral tests. Genes Brain Behav 5:64–72
Li X, Semenova S, D'Souza MS, Stoker AK, Markou A (2014) Involvement of glutamatergic and GABAergic systems in nicotine dependence: Implications for novel pharmacotherapies for smoking cessation. Neuropharmacology 76 Pt B:554–565
Liu C, Cripe TP, Kim MO (2010) Statistical issues in longitudinal data analysis for treatment efficacy studies in the biomedical sciences. Mol Ther 18:1724–1730
Martin-Ruiz CM, Lee M, Perry RH, Baumann M, Court JA, Perry EK (2004) Molecular analysis of nicotinic receptor expression in autism. Brain Res Mol Brain Res 123:81–90
Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM (2007) Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 190:269–319
McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN (2008) Autism-like behavioral phenotypes in BTBR T + tf/J mice. Genes Brain Behav 7:152–163
McTighe SM, Neal SJ, Lin Q, Hughes ZA, Smith DG (2013) The BTBR mouse model of autism spectrum disorders has learning and attentional impairments and alterations in acetylcholine and kynurenic acid in prefrontal cortex. PLoS One 8:e62189
Moser N, Mechawar N, Jones I, Gochberg-Sarver A, Orr-Urtreger A, Plomann M, Salas R, Molles B, Marubio L, Roth U, Maskos U, Winzer-Serhan U, Bourgeois JP, Le Sourd AM, De Biasi M, Schroder H, Lindstrom J, Maelicke A, Changeux JP, Wevers A (2007) Evaluating the suitability of nicotinic acetylcholine receptor antibodies for standard immunodetection procedures. J Neurochem 102:479–492
Newhouse P, Kellar K, Aisen P, White H, Wesnes K, Coderre E, Pfaff A, Wilkins H, Howard D, Levin ED (2012) Nicotine treatment of mild cognitive impairment: a 6-month double-blind pilot clinical trial. Neurology 78:91–101
Nicolson R, Craven-Thuss B, Smith J (2006) A prospective, open-label trial of galantamine in autistic disorder. J Child Adolesc Psychopharmacol 16:621–629
Perry EK, Lee ML, Martin-Ruiz CM, Court JA, Volsen SG, Merrit J, Folly E, Iversen PE, Bauman ML, Perry RH, Wenk GL (2001) Cholinergic activity in autism: abnormalities in the cerebral cortex and basal forebrain. Am J Psychiatry 158:1058–1066
Picciotto MR (2003) Nicotine as a modulator of behavior: beyond the inverted U. Trends Pharmacol Sci 24:493–499
Popke EJ, Mayorga AJ, Fogle CM, Paule MG (2000) Effects of acute nicotine on several operant behaviors in rats. Pharmacol Biochem Behav 65:247–254
Potter AS, Dunbar G, Mazzulla E, Hosford D, Newhouse PA (2014) AZD3480, a novel nicotinic receptor agonist, for the treatment of attention-deficit/hyperactivity disorder in adults. Biol Psychiatry 75:207–214
Preskorn SH, Gawryl M, Dgetluck N, Palfreyman M, Bauer LO, Hilt DC (2014) Normalizing effects of EVP-6124, an alpha-7 nicotinic partial agonist, on event-related potentials and cognition: a proof of concept, randomized trial in patients with schizophrenia. J Psychiatr Pract 20:12–24
Prickaerts J, van Goethem NP, Chesworth R, Shapiro G, Boess FG, Methfessel C, Reneerkens OA, Flood DG, Hilt D, Gawryl M, Bertrand S, Bertrand D, Konig G (2012) EVP-6124, a novel and selective alpha7 nicotinic acetylcholine receptor partial agonist, improves memory performance by potentiating the acetylcholine response of alpha7 nicotinic acetylcholine receptors. Neuropharmacology 62:1099–1110
Radcliffe KA, Fisher JL, Gray R, Dani JA (1999) Nicotinic modulation of glutamate and GABA synaptic transmission of hippocampal neurons. Ann N Y Acad Sci 868:591–610
Ray MA, Graham AJ, Lee M, Perry RH, Court JA, Perry EK (2005) Neuronal nicotinic acetylcholine receptor subunits in autism: an immunohistochemical investigation in the thalamus. Neurobiol Dis 19:366–377
Rezvani AH, Cauley MC, Johnson EC, Gatto GJ, Levin ED (2012) Effects of AZD3480, a neuronal nicotinic acetylcholine receptor agonist, and donepezil on dizocilpine-induced attentional impairment in rats. Psychopharmacology 223:251–258
Shameem M, Patel AB (2012) Glutamatergic and GABAergic metabolism in mouse brain under chronic nicotine exposure: implications for addiction. PLoS One 7:e41824
Silverman JL, Tolu SS, Barkan CL, Crawley JN (2010) Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology 35:976–989
Silverman JL, Smith DG, Rizzo SJ, Karras MN, Turner SM, Tolu SS, Bryce DK, Smith DL, Fonseca K, Ring RH, Crawley JN (2012) Negative allosteric modulation of the mGluR5 receptor reduces repetitive behaviors and rescues social deficits in mouse models of autism. Sci Transl Med 4:131ra51
Silverman JL, Oliver CF, Karras MN, Gastrell PT, Crawley JN (2013) AMPAKINE enhancement of social interaction in the BTBR mouse model of autism. Neuropharmacology 64:268–282
Silverman JL, Pride MC, Hayes JE, Puhger KR, Butler-Struben HM, Baker S, Crawley JN (2015) GABA receptor agonist r-baclofen reverses social deficits and reduces repetitive behavior in two mouse models of Autism. Neuropsychopharmacology
Sparks JA, Pauly JR (1999) Effects of continuous oral nicotine administration on brain nicotinic receptors and responsiveness to nicotine in C57Bl/6 mice. Psychopharmacology 141:145–153
Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C, Jiang T, Zhu XC, Tan L (2014) Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis 41:615–631
Tatem KS, Quinn JL, Phadke A, Yu Q, Gordish-Dressman H, Nagaraju K (2014) Behavioral and locomotor measurements using an open field activity monitoring system for skeletal muscle diseases. Journal of Vis Exp : JoVE: 51785
Wainwright PE, Leatherdale ST, Dubin JA (2007) Advantages of mixed effects models over traditional ANOVA models in developmental studies: a worked example in a mouse model of fetal alcohol syndrome. Dev Psychobiol 49:664–674
White HK, Levin ED (1999) Four-week nicotine skin patch treatment effects on cognitive performance in Alzheimer's disease. Psychopharmacology 143:158–165
Yang M, Abrams DN, Zhang JY, Weber MD, Katz AM, Clarke AM, Silverman JL, Crawley JN (2012) Low sociability in BTBR T + tf/J mice is independent of partner strain. Physiol Behav 107:649–662
Yates SL, Bencherif M, Fluhler EN, Lippiello PM (1995) Up-regulation of nicotinic acetylcholine receptors following chronic exposure of rats to mainstream cigarette smoke or alpha 4 beta 2 receptors to nicotine. Biochem Pharmacol 50:2001–2008
Zikopoulos B, Barbas H (2013) Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front Hum Neurosci 7:609
Acknowledgments
The authors are grateful to LaShon Middleton for expert technical support during experiments. We thank the Children’s Research Institute neurobehavior core for the use of the facility. The work was supported by a grant from the Sheikh Zayed Institute for Pediatric Surgical Innovation, Children’s Research Institute, Children’s National Health System and by a grant from the National Institutes of Health Intellectual & Developmental Disabilities Research Center Grant P30HD040677
Conflict of interest
All authors have read the journal’s authorship agreement and policy on disclosure of potential conflicts of interest and have no conflict of interest to disclose
Author information
Authors and Affiliations
Corresponding author
Additional information
In memoriam of Nicholas Kenyon
Li Wang and Luis E. F. Almeida contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 326 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Almeida, L.E.F., Spornick, N.A. et al. Modulation of social deficits and repetitive behaviors in a mouse model of autism: the role of the nicotinic cholinergic system. Psychopharmacology 232, 4303–4316 (2015). https://doi.org/10.1007/s00213-015-4058-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4058-z