Abstract
The aim of this study was to determine, in the diet-induced obesity model in rats, the potential of Guanabenz to reduce body weight and ameliorate some metabolic disturbances. Obesity was induced in rats by a high-fat diet. After 10 weeks, rats were treated intraperitoneally with Guanabenz at the two doses: 2 or 5 mg/kg b.w./day, once daily for 25 days. The spontaneous activity of rats was measured for 24 h on the 1st and 24th day of the Guanabenz treatment with a special radio-frequency identification system. Gastric emptying was measured in intragastric phenol red-treated mice by measuring the color of the stomach homogenate 30 min after phenol red administration. Intraperitoneal administration of Guanabenz for 25 days to obese rats resulted in a significant decrease in body weight compared to the baseline values (about 11% at a dose of 5 mg/kg). Both body weight and the amount of adipose tissue in the groups receiving Guanabenz decreased to the levels observed in the control rats fed only standard feed. The anorectic effect occurred in parallel with a reduction in plasma triglyceride levels. We also confirmed the beneficial effect of Guanabenz on plasma glucose level. The present study demonstrates that the administration of Guanabenz strongly inhibits gastric emptying (about 80% at a dose of 5 mg/kg). Guanabenz can successfully and simultaneously attenuate all the disorders and risk factors of metabolic syndrome: hypertension, hyperglycemia, obesity, and dyslipidemia. However, the exact cellular mechanisms of its action require further research.
Similar content being viewed by others
Introduction
Every year, many people die of overweight, obesity, and the numerous health conditions that follow excess weight gain, particularly type 2 diabetes mellitus, coronary disorders, or increased incidence of various forms of cancer. Nowadays, obesity is also associated with a higher risk of death among those infected with COVID-19 (Jimenez-Munoz et al. 2021). For health, social, and economic reasons, there is an urgent need to find novel, effective, and safe weight-reducing drugs. On the other hand, it is valuable to search for new indications, among the pool of drugs already registered for other diseases. Such an approach provides us with the knowledge which can be used faster and more efficiently to combat the specific disease.
Guanabenz (2,6-dichlorobenzenelidenamminoaguanidine acetate) is a central sympathetic agent belonging to the class of “aminoguanidine”. Its chemical structure derives from the combination of the substituted benzene ring of clonidine, a non-selective α2-adrenoceptor agonist, with the aminoguanidine side chain of guanethidine (Tu et al. 2017; Hall et al. 1985). Similarly to the other central sympatholytic agents, Guanabenz appears to exert its antihypertensive activity by selectively stimulating the central postsynaptic α2-adrenoceptors located in the brainstem, along with the rostral ventrolateral medulla, the regulatory site of sympathetic activity (Kario 2018; Sica 2007). Central activation of the α2-adrenoceptors leads to a cycle of hemodynamic events resulting in a decrease in systemic blood pressure (Baum and Shropshire 1976; Bosanac et al. 1976).
The hypotensive effects of Guanabenz are reflected in a decrease in norepinephrine levels due to inhibition of sympathetic outflow from the brain to the peripheral system, a reduction in total peripheral resistance, cardiac output, and heart rate both at rest and during exercise. Moreover, in patients treated with Guanabenz, a lower renin release and an increased sodium excretion were detected (Holmes et al. 1983). Guanabenz effectiveness in the regression of left ventricular hypertrophy has also been reported (Miyajima et al. 2000; Vongpatanasin et al. 2011). It may be used safely in antihypertensive therapy due to the absence of acute effects on cardiac function (Walker et al. 1977). In addition, recent studies have highlighted the potential of Guanabenz to lower serum cholesterol levels by approximately 10–20%. This noteworthy metabolic activity may be attributed to inhibition of cholesterol production at the hepatic level, as well as triglyceride synthesis (Kario 2018).
Interaction with α2-adrenoceptors settled in the nucleus tractus solitarii, nucleus coeruleus, and salivary glands frequently brings about side effects such as sedation, dry mouth, and impotence (McMahon et al. 1977).
The onset of the Guanabenz pharmacological action occurs in 1–2 h and persists for approximately 8 up to 12 h, while the plasma peak concentration is achieved within 2–5 h after oral administration (Meacham et al. 1981). It is quickly absorbed into the gastrointestinal tract, subjected to extensive hepatic first-pass metabolism and eliminated in the urine within the first 24 h mainly as the inactive metabolite (E)-p-hydroxyguanabenz (Lasseter et al. 1984). Less than 1% of the dose is excreted as an unchanged drug. Guanabenz is 90% bound to human plasma proteins (Holmes et al. 1983).
The results of our experiments, revealing that Guanabenz may reduce body weight and ameliorate some metabolic disturbances, are highly valuable. The compound has already undergone extensive safety and efficacy trials, so its further development and search for new indications are beneficial not only from the social but also an economic point of view.
Materials and methods
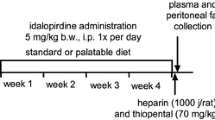
The scheme of the experiment is shown in Fig. 1.
Animals
Experiments were carried out on male Wistar rats (total of fifty-five animals) with an initial body weight of 150–160 g (6-weeks-old — obesity model, rats’ core temperature measurement) or 220–260 g (8-weeks-old — blood pressure test) and on twenty-eight male CD-1 mice with the body weight of 25 g (8-weeks-old — inhibition of gastric emptying). The animals were obtained from the animal house of the Faculty of Pharmacy, Jagiellonian University Medical College, Cracow. The rats were housed in pairs in plastic cages in constant-temperature facilities exposed to a light–dark cycle; water and food were available ad libitum. The randomly established experimental groups consisted of 6 rats or 6 mice. All experiments were carried out according to the guidelines of the Animal Use and Care Committee of the Jagiellonian University and were approved for realization (Permissions No 54/2012 and No 128/2017).
The study was carried out according to the Basic and Clinical Pharmacology and Toxicology policy for experimental and clinical studies (Tveden-Nyborg et al. 2021).
Compounds
Guanabenz acetate (Sigma Aldrich, Germany) was administered intraperitoneally (i.p.) to rats, at the doses of 2 or 5 mg/kg b.w./day (LD50 for rats after i.p. administration is 64 mg/kg b.w. (PubChem [Internet] 2021)). Vehicle (water) was administered i.p. at a volume of 1 ml/kg. This route of administration was chosen to ensure that the special high-fat feed would not interfere with drug absorption. Only in the test of gastric-emptying inhibition, Guanabenz was administered to mice intragastrically (i.g.) according to the available methodology.
Heparin and loperamide were purchased from Polfa Warszawa S.A. (Warsaw, Poland), and thiopental sodium was obtained from Sandoz GmbH (Kundl, Austria).
Induction of obesity with a high-fat diet (HFD) and influence of Guanabenz on body weight, energy intake, and spontaneous activity
Male Wistar rats were fed HFD consisting of 40% fat from blend (Labofeed B with 40% lard, Morawski, Manufacturer Feed, Poland) for 13 weeks, with water available ad libitum (Dudek et al. 2015a; Kotańska et al. 2018). Control rats were fed a standard diet (Labofeed B, Morawski Manufacturer Feed, Poland).
After 10 weeks, rats with obesity were randomly divided into three equal groups that had the same mean body weight and were treated i.p. with Guanabenz at the two doses: 2 or 5 mg/kg b.w./day (two experimental groups) or with vehicle (diet-induced obesity control group) once a day in the morning between 9:00 A.M. and 10:00 A.M. for 25 days. Rats with obesity (total of three groups) were kept on a HFD throughout the treatment period (25 days). Control rats (one group) were maintained on a standard diet and during the last 25 days of the experiment were receiving daily i.p. injections of vehicle (water). Guanabenz was administered i.p. to exclude the potential influence of a special feed on absorption and bioavailability.
Food intake was measured three times a week, and body weight was measured daily, immediately prior to the Guanabenz administration. The feed was given in special feeders specially adapted to rats, which limited its spreading around the cage.
Fatty feed composition (932 g of dry mass): protein — 193 g, fat (lard) — 408 g, fiber — 28.1 g, crude ash — 43.6 g, calcium — 9.43 g, phosphorus — 5.99 g, sodium — 1.76 g, sugar — 76 g, magnesium — 1.72 g, potassium — 7.62 g, manganese — 48.7 mg, iodine — 0.216 mg, copper — 10.8 mg, iron — 125 mg, zinc — 61.3 mg, cobalt — 0.253 mg, selenium — 0.304 mg, vitamin A — 15,000 units, vitamin D3 — 1000 units, vitamin E — 95.3 mg, vitamin K3 3.0 mg, vitamin B1 — 8.06 mg, vitamin B2 — 6.47 mg, vitamin B6 — 10.3 mg, vitamin B12 — 0.051 mg, folic acid — 2.05 mg, nicotinic acid — 73.8 mg, pantothenic acid — 19.4 mg, choline — 1578 mg. High-fat diet (HFD) contained 100 g feed — 550 kcal.
Standard diet contained 100 g feed — 280 kcal.
The spontaneous activity of rats was measured for 24 h on the 1st (after first administration) and 24th (after multiple administrations) day of the Guanabenz treatment at a dose of 5 mg/kg b.w./day with a special radio-frequency identification system (RFID-system) — TraffiCage (TSE-Systems, Germany) (Dudek et al. 2015b, 2016). The animals had subcutaneously implanted transmitter identification, which allowed the presence and time spent in different areas of the cage to be recorded, and then the data were collected with a special computer program.
Collection of the peritoneal fat and plasma
After the last (twenty-fifth) administration of the test compound, the feed was discontinued. On the 26th day of the experiment, 20 min after i.p. administration of heparin (5000 IU/rat) and thiopental (70 mg/kg b.w.), blood was collected from the left carotid artery and then centrifuged at 600 × g (15 min, 4 °C) in order to obtain plasma. Intraperitoneal fat was also collected and weighed.
Biochemical analysis
To determine the levels of glucose, total cholesterol, and triglycerides in plasma, standard enzymatic and spectrophotometric tests (Biomaxima S.A. Lublin, Poland) were used.
Effect of Guanabenz on the rats’ core temperature
Animals with induced obesity (two additional groups of six rats each) were subcutaneously implanted with a DST micro-HRT heart rate logger (Star-Oddi, Island), which simultaneously measured the long-term core temperature. Under general anesthesia (thiopental, 70 mg/kg, i.p.), the loggers were inserted under the skin in the groin area and sutured with surgical thread. Forty-eight hours later, the baseline temperature was measured, and at 9:30 A.M., single Guanabenz dose (5 mg/kg b.w.) was administered i.p. The first measurement was recorded 30 min after the test compound administration, and then the temperature was recorded every hour. After 24 h, the loggers were removed, the temperature data were read, and Mercury software (Star-Oddi Mercury Data Logger PC Software, Iceland) was used to collect and analyze the obtained information (Dudek et al. 2015a). Using this technology, measurements are made without the direct intervention of the researcher; therefore, there is no disturbance in the readings evoked by stress in the animals.
Inhibition of gastric emptying in the mouse model
The experiment was planned in order to evaluate the ability of the test drug to inhibit gut motility and was conducted according to Miyasaka et al. (Miyasaka et al. 2004) with some minor modifications. Mice were deprived of food for 22 h and of water for 2 h prior to the testing. Vehicle (0.25 ml/mouse) and drugs: Guanabenz (5 mg/kg b.w.) or loperamide (reference compound — 10 mg/kg b.w) were administered i.g. by gastric gavage. Phenol red (0.5 ml, 0.05% w/v) was dosed i.g. 45 min later. Seven vehicle-treated mice were sacrificed immediately after phenol red administration and used as a standard control (100% phenol red in the stomach = 0 min), these points were considered to have a maximum absorbance (100%). The remaining mice were sacrificed 30 min later. Stomachs were excised and homogenized (by OMNI homogenizer, OMNI International a PerkinElmer Company, USA) with 10 ml of NaOH (0.1 N). To 5 ml of homogenate, 0.5 ml of 20% w/v trichloroacetic acid was added to precipitate the proteins. Then, samples were centrifuged at 600 × g (15 min, 20 °C), and 1 ml of supernatant was mixed with 4 ml of 0.5 N NaOH to develop the highest color intensity. The level of phenol red was determined by spectrophotometry (absorbance assessed at 560 nm). Gastric emptying (GE %) was calculated for each mouse according to the following equation:
The effect of Guanabenz on blood pressure of non-obese rats (Kotańska et al. 2018)
Eighteen normotensive rats were anesthetized with thiopental (70 mg/kg) by i.p. injection. The left carotid artery was cannulated with tubing filled with heparin solution in saline to facilitate pressure measurements using Apparatus PowerLab 4/35 (ADInstruments, Australia). Blood pressure was measured: before i.p. administration of Guanabenz at the doses of 2 or 5 mg/kg b.w. or water — time 0 min (control pressure) and continuously during the next 60 min.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6 software (GraphPad Software, USA). The data should be normally distributed to perform the analysis of variance; therefore, normality of the datasets was determined using the Shapiro–Wilk test. Results showed that the analysis of variance was appropriate, and comparisons between experimental and control groups were performed by one-way or two-way ANOVA, followed by Tukey post hoc or multiple t tests (spontaneous activity). Differences were considered significant at p ≤ 0.05.
Results
Calorie intake
In the first week of Guanabenz administration (regardless of the dose administered), a significant decrease in kcal intake was observed by treated animals compared to the obese control group. The effect was less pronounced after administration of Guanabenz at a dose of 2 mg/kg b.w.; therefore, the kcal intake in these animals was significantly higher than in the control group fed only standard feed. However, in the second and third weeks of treatment, only animals in the group receiving the test compound at a dose of 5 mg/kg b.w. consumed significantly less kcal compared to the obese control group (F(6,16) = 22.28, p < 0.0001) (Fig. 2).
Effect of Guanabenz administration on calorie intake in obese rats Results are expressed as means ± SEM, n = 3. Comparisons were done by two-way ANOVA, Tukey’s post hoc tests. *control group fed palatable diet vs. control group fed standard diet; ^Guanabenz administered at a dose of 2 mg/kg b.w. group vs. control group fed standard diet; •Guanabenz administered at a dose of 2 mg/kg b.w. group vs. control group fed HFD diet; †Guanabenz administered at a dose of 5 mg/kg b.w. group vs. control group fed HFD diet; ^^p < 0.01; ***, ^^^, †††, •••p < 0.001
Gastric emptying
Guanabenz administered at a dose of 5 mg/kg b.w., i.g. statistically significantly delayed gastric emptying (F(2, 17) = 74.45, p < 0.001) (Fig. 3). This effect was even stronger than after administration of the reference compound — loperamide (10 mg/kg b.w., i.g.).
Effect of Guanabenz administration on gastric emptying. Results are presented as box plots showing the following data: mean (“ + ”), median (horizontal line), upper and lower quartile (the width of the box shows interquartile range), upper and lower extreme (whiskers). Statistical analysis: Shapiro–Wilk test for normality and one-way ANOVA (Tukey post hoc), n = 6–7; *significant against control group (vehicle); ^significant against Loperamide at a dose of 10 mg/kg b.w. administered group; ^p < 0.05; ***p < 0.001
Spontaneous activity
Monitoring spontaneous activity in obese animals showed a sedative effect after the first administration of Guanabenz at a dose of 5 mg/kg b.w. At several time points, the reduction in activity was significant compared to that observed in rats from the obese control group. However, during the chronic treatment, significant sedation was observed only on the 1st, 4th, and 23rd hour after the last (24th) administration (Fig. 4).
Body weight and amount of peritoneal fat
The i.p. administration of Guanabenz for 25 days at a dose of 2 or 5 mg/kg b.w./day to obese rats resulted in a significant decrease in body weight by 1.94% and 11.06%, respectively, compared to the baseline values (F(3, 20) = 37.35, p < 0.0001) (Fig. 5b). Simultaneously, less amount of peritoneal adipose tissue was observed, about 41.30% and 64.77%, in groups treated with Guanabenz compared to the amount determined in control obese rats (F(3, 20) = 25.45, p < 0.0001) (Fig. 5c). At the same time, the control rats fed HFD, gained an additional 9.81% of weight, while rats from control group fed standard feed increased their body weight by 6.80% (they grew). Both body weight (F(36, 240) = 18.67, p < 0.001) and the amount of adipose tissue in the groups receiving Guanabenz decreased to the levels observed in the control rats fed only standard feed (Fig. 5a and c).
Effect of Guanabenz administration on body weight (a), sum of body weight changes (b) or mass of adipocyte pads (c) in obese rats. a Results are expressed as means ± SEM, n = 6. Multiple comparisons were done by two-way ANOVA, Tukey’s post hoc tests. b, c Results are presented as box plots showing the following data: mean ( +), median (horizontal line), upper and lower quartile (the width of the box shows interquartile range), upper and lower extreme (whiskers). Statistical analysis: Shapiro–Wilk test for normality and one-way ANOVA (Tukey post hoc), n = 6; *control group fed palatable diet vs. control group fed standard diet; ^Guanabenz administered at a dose of 2 mg/kg b.w. group vs. control group fed standard diet; + Guanabenz administered at a dose of 5 mg/kg b.w. group vs. control group fed standard diet; •Guanabenz administered at a dose of 2 mg/kg b.w. group vs. control group fed HFD diet; †Guanabenz administered at a dose of 5 mg/kg b.w. group vs. control group fed HFD diet; ^,•,†, + p < 0.05; ••, ††p < 0.01; ***,†††,•••, + + + p < 0.001
Plasma levels of glucose, triglycerides and total cholesterol
Guanabenz statistically significantly decreased glucose levels (F(3, 20) = 8.337, p = 0.0009) and triglycerides (F (3, 19) = 3.321, p = 0.0419) in obese rats (Fig. 6a and b). The obese control group had significantly higher triglyceride levels compared to the control group fed only standard feed, and after treatment with Guanabenz (regardless of the dose), there were no significant difference in triglyceride levels compared to the control group fed only standard feed (Fig. 6b). Total cholesterol was significantly higher in all groups fed HFD compared to the level determined in the group fed standard feed (F(3, 20) = 12.04, p = 0.0001) (Fig. 6c).
Effect of Guanabenz administration on plasma levels of glucose (a), triglyceride (b), total cholesterol (c) in obese rats. Results are presented as box plots showing the following data: mean ( +), median (horizontal line), upper and lower quartile (the width of the box shows interquartile range), upper and lower extreme (whiskers). Statistical analysis: Shapiro–Wilk test for normality and one-way ANOVA (Tukey post hoc), n = 5–6; *control group fed palatable diet vs. control group fed standard diet; ^Guanabenz administered at a dose of 2 mg/kg b.w. group vs. control group fed standard diet; + Guanabenz administered at a dose of 5 mg/kg b.w. group vs. control group fed standard diet; •Guanabenz administered at a dose of 2 mg/kg b.w. group vs. control group fed HFD diet; †Guanabenz administered at a dose of 5 mg/kg b.w. group vs. control group fed HFD diet; *,^,†p < 0.05; **,^^p < 0.01; ***, + + + p < 0.001
Core body temperature
Guanabenz at a dose of 5 mg/kg b.w. caused a significant reduction in core temperature, but only from 7 to 14th h after administration (F(23, 230) = 14.26, p < 0.0001). The maximum decrease in temperature was observed at the 9th h after Guanabenz administration at a dose of 5 mg/kg b.w (Fig. 7).
Blood pressure
The effect on blood pressure was determined in normotensive, non-obese rats. Significant changes were observed only on the 5th (increase) and 10th min (decrease) after i.p. administration of Guanabenz at a dose of 5 mg/kg b.w. (F(16,128) = 3.081, p = 0.0002 — systolic; F(16,128) = 3.931, p < 0.001 — distolic). For the remaining time of the experiment, blood pressure of rats treated with Guanabenz (both doses) was slightly lower than in control animals; however, differences were not statistically significant (Fig. 8).
Effect of administration of Guanabenz on blood pressure. Results are expressed as means ± SEM, n = 6–7. Comparisons were done by two-way ANOVA, Tukey’s post hoc tests. *control group vs. Guanabenz administered at a dose of 5 mg/kg b.w. group — systolic pressure; ^control group vs. Guanabenz administered at a dose of 5 mg/kg b.w. group —diastolic pressure; ^p < 0.05; **p < 0.01
Discussion
In our study, we have shown that chronic administration of Guanabenz causes not only a significant decrease in body weight of animals with developed obesity, but also, significant decrease in the blood levels of glucose and triglycerides. Since Guanabenz is a long-acting agonist of α2-adrenoceptors (Kario 2018), it occupies these receptors preventing their rapid stimulation by successive portions of noradrenaline. Additionally, Guanabenz blocks α1-adrenoceptors (Takeuchi et al. 1987), so it can also, indirectly, lead to an increase in noradrenaline binding to β-adrenoreceptors (e.g., β3-adrenoreceptor). These effects may be related to its weight reducing activity and the absence of bradycardia after repeated administrations. There are three main categories of weight loss by (1) decreasing energy intake by suppressing appetite, (2) reducing energy intake by impaired absorption, and (3) increasing energy expenditure (Jimenez-Munoz et al. 2021). The aim of our research was to pre-define into which of these categories falls the effect of Guanabenz after its repeated administration to obese rats. The results will allow us to narrow down the search for the exact mechanisms of Guanabenz’s activity in obesity for future studies.
It is known that obesity is a major cause of insulin resistance, impaired glucose tolerance, type II diabetes, elevated plasma concentrations of triglycerides and low-density cholesterol, decreased plasma concentrations of high-density cholesterol and hypertension (Pi-Sunyer 2002). Therefore, it is an important finding that in obese animals treated with Guanabenz, an anorectic effect occurred in parallel with lowering plasma triglyceride levels. It is common knowledge that a decrease in food intake leads to increased lipolysis, and hence, to the hydrolysis of triglycerides in adipocyte lipid droplets (Gogga et al. 2011). In our studies, Guanabenz administered at a dose of 5 mg/kg b.w. had an inhibitory effect on appetite that may be associated with increased lipolysis and body weight reduction. Previous studies reported that chronic use of Guanabenz caused a small decrease in serum cholesterol levels (Holmes et al. 1983; Kaplan 1984). Our research did not show such an effect of Guanabenz, but we only measured total cholesterol, which is one of the limitations of our study.
Additionally, in our research, we confirmed, described in the literature, the beneficial effect of Guanabenz on the plasma glucose level. Previously, Guanabenz was shown in mice to cause an increase in blood glucagon-like peptide 1 (GLP-1) and insulin levels, a decrease in blood glucose level, and an elevation of leptin level together with a reduction in food intake (Ye et al. 2013). Yoshino and co-workers showed that a 14-day administration of Guanabenz to obese mice compensated for glucose and triglyceride levels by influencing hepatic metabolism (Yoshino et al. 2020). In our studies, we showed the hypoglycemic effect of Guanabenz administration in the obesity state induced by diet, what may also be associated with reduced food intake.
Gastric motility is a key mediator of hunger and satiety. Gastric accommodation and emptying (the passage of the contents from the stomach to the next parts of the intestine) play an important role in the regulation of gastric (dis)tension and intestinal exposure of nutrients, and therefore control satiation, and can play a role in the long-term regulation of body weight (Janssen et al. 2011). There are no studies in the literature to explore the effect of Guanabenz on gastric emptying. To the best of our knowledge, there is only one report (preclinical studies) that describes that the administration of Guanabenz resulted (in dogs) in an inhibition of the gastrointestinal motility (Ohata et al. 1983). Therefore, the present study is the first one to demonstrate that the administration of Guanabenz at a dose of 5 mg/kg b.w. inhibits gastric emptying to a greater extent than the reference compound — loperamide — administered at the dose of 10 mg/kg b.w. The dose of loperamide was chosen based on the study in which, at this particular dose, it reduced gastric emptying in mice in a similar test (Matsumoto et al. 2008; Salako et al. 2015 Dec; Armstrong et al. 2013). The delay in gastric emptying can be associated with weight loss, as reported in the case of liraglutide (analog of GLP-1) (Halawi et al. 2017; Can et al. 2014). Since, Guanabenz in mice was shown to cause an increase in the blood GLP-1, further studies investigating the effect of Guanabenz on gastrointestinal motility and excretion should be performed to determine the relationship between its incretin mimetic effect, its ability to inhibit gastric emptying, and its effect on body weight, as well as to reveal the cellular mechanisms of this action (Ye et al. 2013).
Previous studies have demonstrated the thermogenic activity of α2-adrenoceptor agonists (Bill et al. 1989). In our previous studies on the effectiveness of Guanfacine (another long-acting, partial α2A-adrenoceptor agonist) in reducing obesity, we showed that it lowers body temperature for the first 3 h after administration. Furthermore, in rats given Guanfacine, significant sweating was observed, which may suggest intensified thermogenesis (Dudek et al. 2015a). In the present study, we clearly show that Guanabenz reduces core temperature; however, this effect is only seen 7 h and later after administration. Therefore, this is a significant difference in the onset of the hypothermic effect induced by Guanabenz and Guanfacine.
Thermoregulation is the maintenance of a relatively constant core body temperature. Abnormal core temperature, even a couple of degrees, activates the body’s thermoregulatory mechanisms. Thus, the heat loss from the body must equal the heat gain. The optimal core temperature of the organism is achieved principally through heat production as a result of metabolism (Tansey and Johnson 2015; Tan and Knight 2018). When body temperature decreases (under the influence of, e.g., drug), the metabolic rate (heat production) increases to keep the temperature within the proper range. The opposite happens during the physical exercise, which in itself causes an increase in the basal temperature (also by increasing metabolism), so then, the organism has to lower the temperature through other mechanisms to maintain homeostasis.
In our study, when the temperature was lowered by Guanabenz administration (e.g., through peripheral vasodilation), the energy to raise the temperature back to normal had to be produced (e.g., by increased metabolism or other). Thus, it seems logical that lowering the temperature by administration of Guanabenz may lead to an increase in metabolism in order to increase the core temperature. However, our study cannot answer the question of whether the temperature changes caused by the administration of Guanabez have any influence on the metabolism rate and subsequently the body weight of experimental animals. It might be interesting to perform a detailed study of such a correlation.
Guanabenz is extensively metabolized and large amounts of metabolites are recovered in the urine in the first 24 h. Less than 1% of the dose is excreted as an unchanged drug, the highest proportion being in the form of the inactive metabolite (E)-p-hydroxyguanabenz (Holmes et al. 1983). The body temperature–lowering effect could be due not only to Guanabenz itself but also or maybe mainly to its metabolites, and this is another issue that needs to be explored.
The weight-reducing effect of Guanabenz took place very quickly, and after about 2 weeks, previously obese animals have lost so much weight that it was no longer different from the slim control animals. Disorders of spontaneous activity (both increase and sedation) and stress, induced by the test compound administration, could affect an assessment of Guanabenz impact on body weight (Dudek et al. 2015b). Moreover, such a rapid normalization of body weight may indicate a toxic effect. Therefore, we monitored the spontaneous activity (if the animals feel ill, the disturbance of spontaneous activity can be considered as a measurable indicator of such state) and measured the levels of corticosterone in the plasma of experimental animals to verify, to some extent, whether the administration of Guanabenz could be harmful. The spontaneous activity of the experimental animals was monitored after both, the first (1st day) and chronic (24th day) administration of the test compound along with the determination of its effect on the body weight. The key was the non-invasive telemetry method, which allows for many hours of data recording in animals pair-housed in home cages, without exposing them to any additional stress and contact with the researcher. We noticed that at some time points after administration, Guanabenz causes sedation; therefore, it should be taken into account that the observed anorectic effect could be partially associated with a reduction in spontaneous activity of animals. However, it should also be mentioned that the reduction in body weight occurred in obese animals (and only to the level observed in rats without obesity), and it was proportional to the reduction in body fat. The lower dose applied, 2 mg/kg b.w., was also effective in reducing body weight; the effect was more subdued, but still noticeable (Fig. 5b).The changes in spontaneous activity occurring after the administration of Guanabenz at certain intervals may also be related to the poor mood of the animals, which is not a desirable effect.
Further studies should be carried out to assess the potential toxicity of Guanabenz, as well as its effectiveness in reducing body weight in obese animals after chronic administration of lower doses (lower dose administered for the longer time may result in less intense but still significant effect). Such studies could also be aimed at the search for a specific mechanism of Guanabenz action.
For the study of the Guanabenz effect on blood pressure, the same doses and route of administration as in the other tests were selected. The aim of this experiment was to determine if these specific doses would cause hypotension in rats under our laboratory conditions. Unfortunately, we were unable to monitor blood pressure using the telemetry system; therefore, measurements were performed for an hour after Guanabenz administration. The lower dose did not have a significant effect, although blood pressure tended to decrease over time. The higher dose caused a significant change in blood pressure in the first minutes after administration, but over time, the difference was not significant from the pressure registered in control animals. Guanabenz is known to produce a biphasic response in systemic blood pressure; an initial dose-dependent increase lasting 4 to 5 min is followed by a prolonged secondary decrease and a toned-down drop in blood pressure after Guanabenz administration is caused by a reduction in vascular resistance (Holmes et al. 1983). Such effect was also noticed in our research. Since we measured blood pressure only for 1 h, it is possible that a longer measurement (using a different method) would show a significant prolonged decrease in this parameter. The decrease in blood pressure is beneficial for overweight and obese people because it reduces the risk of numerous complications related to excess body weight and lipid disorders, and it also relieves the heart and reduces its oxygen demand. Therefore, Guanabenz undoubtedly exerts a very good combination of pharmacological effects considering its benefits in reducing obesity and hypotensive activity.
The following are the limitations of this study: (i) Body weight was not uniform between the tested groups at the beginning of the treatment period. The animals were randomly assigned to experimental groups, and at the time of initiation of Guanabenz or vehicle administration, there were no statistically significant differences in body weight between the groups. Looking at the results presented in Fig. 5b, the difference in initial body weight was not of great importance in determining the effect of Guanabenz. The weight loss is undeniable. Nevertheless, in subsequent experiments, we will pay special attention to ensure that the initial parameters such as body weight are uniform between the tested groups. (ii) A small group of data (n = 3) was used to calculate the food intake after Guanabenz administration. This is undoubtedly a significant limitation of this study, although the results were consistent and the differences were statistically significant. The number of animals for each experiment was selected based on previous studies (Dudek et al. 2015b; Kotańska et al. 201). In preliminary pharmacological studies (such as this one) committing a type 1 error, i.e., declaring the effect when it actually does not exist, is less disadvantageous than committing a type 2 error (not rejecting a false null hypothesis, i.e., stating no effect when it exists). (iii) The measurement of only total cholesterol in plasma. The determination of the concentration of total cholesterol in the plasma gives information about the sum of the individual cholesterol fractions: LDL (low-density lipoprotein), HDL (high-density lipoprotein), and other intermediate fractions. Typically, in metabolic disorders, an increase in total cholesterol levels coincides with an increase in LDL concentration. However, it is also possible that under the influence of some factors, the level of HDL fraction significantly increases, which translates into increased concentration of the total cholesterol in plasma. When total cholesterol concentration increases, but simultaneously triglyceride concentration decreases, we can speculate that the increased cholesterol levels could be due to the high concentration of HDL fraction, which, however, should be confirmed in the further studies. High HDL cholesterol levels are inversely related to the incidence of cardiovascular events. Additionally, HDL is also believed to exhibit anti-inflammatory, antioxidant, fibrinolytic, and anticoagulant effects, possibly directly counteracting the complications of atherosclerosis. Therefore, HDL cholesterol shows a beneficial, multidirectional pleiotropic effect (Kashyap 1998), and an increase in its plasma concentration should be considered beneficial. In conclusion, we obtained a metabolically beneficial effect of Guanabenz on the lipid and carbohydrate profile. However, the determination of the exact mechanism of this effect and the actual share of HDL cholesterol fraction in the total cholesterol pool requires further research.
Conclusion
Our preliminary research shows that Guanabenz is a good candidate as an obesity-reducing drug. We showed that it reduces body weight and has a beneficial influence on glucose and triglyceride levels in obese rats. These effects may be associated with a reduction in caloric intake or/and delay in gastric emptying. Therefore, it is a drug that can successfully and simultaneously attenuate all of the disorders and risk factors of the metabolic syndrome: hypertension, hyperglycemia, obesity, and dyslipidemia. The exact cellular mechanisms of its action require further research.
Data availability
The original source data has been included in the "Supplemental data".
References
Armstrong SR, Campbell CB, Richardson CL, Vickery RG, Tsuruda PR, Long DD, Hegde SS, Beattie DT (2013) The in vivo pharmacodynamics of the novel opioid receptor antagonist, TD-1211, in models of opioid-induced gastrointestinal and CNS activity. Naunyn Schmiedebergs Arch Pharmacol 386(6):471–478
Baum T, Shropshire AT (1976) Studies on the centrally mediated hypotensive activity of guanabenz. Eur J Pharmacol 37(1):31–44
Bill DJ, Hughes IE, Stephens RJ (1989) The termogenic actions of α2-adrenoreceptor agonists in reserpinized mice are mediated via central postsynaptic α2-adrenoreceptor mechanism. Br J Pharmacol 96:133–143
Bosanac P, Dubb J, Walker B, Goldberg M, Agus ZS (1976) Renal effects of Guanabenz: a new antihypertensive. J Clin Pharmacol 16:631–636. https://doi.org/10.1002/j.1552-4604.1976.tb01502.x
Dudek M, Knutelska J, Bednarski M, Nowiński L, Zygmunt M, Mordyl B, Głuch-Lutwin M, Kazek G, Sapa J, Pytka K (2015a) A comparison of the anorectic effect and safety of the alpha2-adrenoceptor ligands guanfacine and yohimbine in rats with diet-induced obesity. PLoS ONE 10(10):e0141327
Dudek M, Marcinkowska M, Bucki A, Olczyk A, Kołaczkowski M (2015b) Idalopirdine – a small molecule antagonist of 5-HT6 with therapeutic potential against obesity. Metab Brain Dis 30:1487–1494
Dudek M, Kuder K, Kołaczkowski M, Olczyk A, Żmudzka E, Rak A, Bednarski M, Pytka K, Sapa J, Kieć-Kononowicz K (2016) H3 histamine receptor antagonist pitolisant reverses some subchronic disturbances induced by olanzapine in mice. Metab Brain Dis 31(5):1023–1029
Gogga P, Karbowska J, Meissner W, Kochan Z (2011) Role of leptin in the regulation of lipid and carbohydrate metabolism. Postepy Hig Med Dosw 65:255–262
Halawi H, Khemani D, Eckert D, O’Neill J, Kadouh H, Grothe K, Clark MM, Burton DD, Vella A, Acosta A, Zinsmeister AR, Camilleri M (2017) Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol 2(12):890–899
Hall AH, Smolinske SC, Kulig KW, Rumack BH (1985) Guanabenz overdose. Ann Intern Med 102:787–788. https://doi.org/10.7326/0003-4819-102-6-787
Holmes B, Brogden RN, Heel RC, Speight TM, Avery GS (1983) Guanabenz. A review of its pharmacodynamic properties and therapeutic efficacy in hypertension. Drugs 26:212–229
Janssen P, Vanden Berghe P, Verscheren S, Lehmann A, Depoortere I, Tack J (2011) Review article: the role of gastric motility in the control of food intake. Aliment Pharmacol Ther 33:880–894
Jimenez-Munoz CM, López M, Albericio F, Makowski K (2021) Targeting energy expenditure – drugs for obesity treatment. Pharmaceuticals 14:435. https://doi.org/10.3390/ph14050435
Kaplan NM (1984) Effects of guanabenz on plasma lipid levels in hypertensive patients. J Cardiovasc Pharmacol 6(Suppl 5):S841–S846
Kario K (2018) Central sympathetic agents and direct vasodilators. Hypertension: A Companion to Braunwald’s Heart Disease, 3rd ed. Elsevier, p 254–260. https://doi.org/10.1016/B978-0-323-42973-3.00026-3
Kashyap ML (1998) Mechanistic studies of high-density lipoproteins. Am J Cardiol 82:42–48
Kotańska M, Śniecikowska J, Jastrzębska-Więsek M, Kołaczkowski M, Pytka K (2017) Metabolic and cardiovascular benefits and risks of EMD386088-A 5-HT6 receptor partial agonist and dopamine transporter inhibitoR. Front Neurosci 8(11):50. https://doi.org/10.3389/fnins.2017.00050.eCollection2017
Kotańska M, Kulig K, Marcinkowska M, Bednarski M, Malawska K, Zaręba P (2018) Metabolic benefits of 1-(3-(4-(o-tolyl)piperazin-1-yl)propyl)pyrrolidin-2-one: a non-selective α-adrenoceptor antagonist. J Endocrinol Invest 41(5):609–619
Lasseter KC, Shapse D, Pascucci VL, Chiang ST (1984) Pharmacokinetics of guanabenz in patients with impaired liver function. J Cardiovasc Pharmacol 6:S766–S770
Matsumoto K, Kimura H, Tashima K, Uchida M, Horie S (2008) Validation of 13C-acetic acid breath test by measuring effects of loperamide, morphine, mosapride, and itopride on gastric emptying in mice. Biol Pharm Bull 31(10):1917–1922
McMahon FG, Ryan JR, Jain AK, Vargas R, Vanov SK (1977) Guanabenz in essential hypertension. Clin Pharmacol Ther 21:272–277. https://doi.org/10.1002/cpt1977213272
Meacham RH, Chiang ST, Kick CJ, Sisenwine SF, Jusko WJ, Ruelius HW (1981) Pharmacokinetic disposition of guanabenz in the rhesus monkey. Drug Metab Dispos 9:509–514. http://dmd.aspetjournals.org/content/9/6/509.abstract
Miyajima E, Shigemasa T, Endo T, Hishiki S, Kawano Y (2000) Guanabenz combination therapy inhibits sympathetic nerve activity and regresses left ventricular hypertrophy. CardiovascDrugs Ther 14:61–66
Miyasaka K, Ohta M, Kanai S, Yoshida Y, Sato N, Nagata A, Matsui T, Noda T, Jimi A, Takiguchi S, Takata Y, Kawanami T, Funakoshi A (2004) Enhanced gastric emptying of a liquid gastric load in mice lacking cholecystokinin-B receptor: a study of CCK-A, B, and AB receptor gene knockout mice. J Gastroenterol 39(4):319–323. https://doi.org/10.1007/s00535-003-1297-2
Ohata K, Murata T, Sakamoto H, Kohno S, Hojo M, Yoshida Y, Nagasaka Y, Akimoto Y, Shimada A, Teramoto N, Tatsumi H (1983) Pharmacological studies of guanabenz. Effects on the peripheral nervous and other organ systems. Nihon Yakurigaku Zasshi 81(1):59–78
Pi-Sunyer XF (2002) The obesity epidemic: pathophysiology and consequences of obesity. Obes Res 10(Suppl 2):97S-104S. https://doi.org/10.1038/oby.2002.202
PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 5702062, Guanabenz acetate; [cited 2021 June 29]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Guanabenz-acetate
Salako OA, Akindele AJ, Shitta OM, Elegunde OO, Adeyemi OO (2015) Antidiarrhoeal activity of aqueous leaf extract of Caladium bicolor (Araceae) and its possible mechanisms of action. J Ethnopharmacol 24(176):225–231
Sica DA (2007) Centrally acting agents. Comprehensive Hypertension, 1st ed. Elsevier, p 1027–1035. https://doi.org/10.1016/B978-0-323-03961-1.50086-6
Takeuchi K, Kogure M, Hashimoto T (1987) Comparison of agonistic and antagonistic action of guanabenz and guanfacine on α1 and α2-adrenoreceptors in isolated smooth muscles. Japan J Pharmacol 43:267–275
Tan CL, Knight ZA (2018) Regulation of body temperature by the nervous system. Neuron 98(1):31–48
Tansey EA, Johnson CD (2015) Recent advances in thermoregulation. Adv Physiol Educ 39(3):139–148
Tu Y, Piascik M, Abel PW (2017) Adrenergic agonists. Pharmacology and Therapeutics for Dentistry, 7th ed. Elsevier, p 110–121. https://doi.org/10.1016/B978-0-323-39307-2.00008-4
Tveden-Nyborg P, Bergmann TK, Jessen N, Simonsen U, Lykkesfeldt J (2021) Polityka BCPT dotycząca badań eksperymentalnych i klinicznych. Basic Clin Pharmacol Toxicol 128:4–8
van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH (2014) Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (lond) 38(6):784–793
Vongpatanasin W, Kario K, Atlas SA, Victor RG (2011) Central sympatholytic drugs. J Clin Hypertens 13:658–661. https://doi.org/10.1111/j.1751-7176.2011.00509.x
Walker BR, Shah RS, Ramanathan KB, Vanov SK, Helfant RH (1977) Guanabenz and methyldopa on hypertension and cardiac performance. Clin Pharmacol Ther 22:868–874. https://doi.org/10.1002/cpt1977226868
Ye H, Charpin-El Hamri G, Zwicky K, Christen M, Folcher M, Fussenegger M (2013) Pharmaceutically controlled designer circuit for the treatment of the metabolic syndrome. Proc Natl Acad Sci U S A 110(1):141–146
Yoshino S, Iwasaki Y, Matsumoto S, Satoh T, Ozawa A, Yamada E, Kakizaki S, Trejo JAO, Uchiyama Y, Yamada M, Mori M (2020) Administration of small-molecule guanabenz acetate attenuates fatty liver and hyperglycemia associated with obesity. Sci Rep 10(1):13671
Acknowledgements
Figure 1 was created with BioRender.com.
Funding
The project was financially supported by National Science Center, Poland—grant no 2011/03/B/NZ7/00635.
Author information
Authors and Affiliations
Contributions
MK conceived and designed research and conducted all experiments. JK conducted some experiments. MK, NN, KM, MS analyzed data and wrote the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants performed by any of the authors.
All applicable international laws for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice in which the studies were conducted.
All experiments were carried out according to the guidelines of the Animal Use and Care Committee of the Jagiellonian University and were approved for realization (Permissions No 54/2012 and No 128/2017).
Consent to participate
Not applicable.
Consent for publication
The authors declare that all data were generated in-house and that no paper mill was used.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kotańska, M., Knutelska, J., Nicosia, N. et al. Guanabenz—an old drug with a potential to decrease obesity. Naunyn-Schmiedeberg's Arch Pharmacol 395, 963–974 (2022). https://doi.org/10.1007/s00210-022-02251-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-022-02251-1