Abstract
Methanol-induced optic neuropathy (Me-ION) is a serious condition that may result in long-term or irreversible visual impairment or even blindness secondary to damage and loss of function of the optic nerve and retina. Me-ION shows a tendency to occur as mass poisonings around the world with a clear predilection for poor societies in developing countries. The main mechanism underlying the molecular basis of Me-ION is the inhibition of the mitochondrial oxidative phosphorylation process through the binding of the toxic metabolite of methanol—formic acid—with the key enzyme of this process—cytochrome c oxidase. However, other mechanisms, including damage to the eye tissues by oxidative stress causing the intensification of the oxidative peroxidation process with the formation of cytotoxic compounds, as well as an increase in the synthesis of pro-inflammatory cytokines and influence on the expression of key proteins responsible for maintaining cell homeostasis, also play an important role in the pathogenesis of Me-ION. Histopathological changes in the eye tissues are mainly manifested as the degeneration of axons and glial cells of the optic nerve, often with accompanying damage of the retina that may involve all its layers. Despite the development of therapeutic approaches, persistent visual sequelae are seen in 30–40% of survivors. Thus, Me-ION continues to be an important problem for healthcare systems worldwide.
Similar content being viewed by others
Introduction
Methanol poisoning is a serious life-threatening condition with a mortality rate ranging from 18 to 44% (Noor et al. 2020). Annually in the US, about 5.000 cases are diagnosed with an incidence of 6.4 cases per 1 million hospitalizations, and with the mean age of patients being 38 ± 18 years with a distinct predominance of the male sex (70 vs. 30%) (Kaewput et al. 2021; Kraut 2016). According to statistical data, the prevalence of methanol intoxication is most common in developing countries and Southeast Asia, mainly among the lower economic and social strata (Abrishami et al. 2011; Noor et al. 2020), and is usually associated with accidental oral consumption due to similar physical properties to ethanol, especially in countries with alcohol prohibition, where a higher percentage of methanol poisonings is observed secondary to the oral consumption of contaminated alcohol from illegal domestic production (Khalili et al. 2021). In the US, unintentional consumption of methanol is the cause of 90% of poisonings, while among cases involving intentional ingestion, suicide attempts slightly predominate (Chung et al. 2018). Another important circumstance is occupational exposure—cases of chronic percutaneous (İşcan et al. 2013) and inhalational (Ma et al. 2019; McCormick et al. 1990; Zhao et al. 2015) exposure to methanol leading to clinically significant consequences have been well documented.
Poisoning with methanol and the product of its metabolism—formic acid—is not harmful to non-primates. However, in primates, including humans, the toxic effect due to ineffective metabolism of formic acid is present (Eells et al. 1981; Fu et al. 2017). The first report of methanol toxicity to the visual system was published by MacFarlan in 1855 (MacFarlan 1855). Methanol-induced optic neuropathy (Me-ION) is a serious condition that can result in long-term or even irreversible visual impairment secondary to damage and loss of function of the optic nerve; however, in many cases, a simultaneous disturbance of other structures—the retina, as well as chiasms and the optic tract is present (Grzybowski et al. 2015). According to estimates, visual symptoms occur in about 50% of the cases of methanol poisoning (Grzybowski et al. 2015; Klein et al. 2017; Seme et al. 2001). The exact dose causing pathological changes in the human visual system have not been precisely determined, and in most cases, it may depend on individual metabolic predisposition; however, it is known that consuming as little as 4 ml of methanol can lead to complete loss of vision (Bennett et al. 1953). Massive outbreaks of toxic optic neuropathy (TON) associated with acute or chronic methanol poisoning occurred in the last century in the 1950s in the US (Bennett et al. 1953; Benton and Calhoun 1953) and in the 1990s in Cuba (Lincoff et al. 1993; Sadun et al. 1994), as well as in recent decades in the El Salvador (Hassanian-Moghaddam et al. 2015), Norway (Hovda et al. 2005), Estonia (Paasma et al. 2007), Czech Republic (Zakharov et al. 2014), Libya (Rostrup et al. 2016), Kenya (Rostrup et al. 2016), Uganda (Doreen et al. 2020), Turkey (Gulen et al. 2020), India (Kumar et al. 2019), and Tunisia (Brahmi et al. 2007). Furthermore, in recent months, changes in social functioning caused by the COVID-19 pandemic have also contributed to a significant increase in the incidence of optic neuropathy related to methanol poisoning reported in various regions of the world including Iran (Khalili et al. 2021; Sefidbakht et al. 2020), as well as the US (Yip et al. 2020).
Despite the development of diagnostic methods, prompt diagnosis, and implementation of appropriate treatment, Me-ION is still a challenge for clinicians due to its severe health consequences that significantly reduce the quality of life of survivors many years after discharge (Rulisek et al. 2020), as well as the economic burden on health systems (Barták et al. 2021)—this issue continues to be a major health care problem worldwide. In this review, we attempt to analyze and discuss the current state of knowledge regarding the pathophysiology, clinical presentation, diagnosis, treatment, as well as prognosis for Me-ION.
Systemic effects of methanol intoxication
Primates, including humans, in contrast to non-primates, are sensitive to methanol due to the limited ability to rapidly metabolize and eliminate methanol and its metabolites from the body (Eells et al. 2000; Plaziac et al. 2003). Formic acid inhibits oxidative phosphorylation by binding to a key enzyme in the mitochondrial respiratory chain—cytochrome c oxidase—causing intracellular adenosine triphosphate (ATP) deficiency (Ahiskali et al. 2019; Saoudi et al. 2011). This effect was observed in both in vitro and in vivo studies at the concentration of formic acid ranging from 5 to 30 nM (Treichel et al. 2003). Disruption in mitochondrial electron transport and production of ATP results in the intensification of anaerobic metabolism with a secondary accumulation of formate and lactate in tissues and the development of metabolic acidosis with secondary serious systemic complications (Gabay et al. 2018; Treichel et al. 2003). Due to the high molar concentration, methanol causes a marked serum osmolal gap, while the anion gap and metabolic acidosis result from the accumulation of formate and lactate in the serum (Hovda et al. 2004). Importantly, as blood pH decreases, the concentration of non-ionized methanol increases, which determines its increased penetration into tissues and a more extensive range of their damage (Garner et al. 1995). Moreover, animal studies have shown that despite almost complete metabolism and removal of methanol from the body, the concentration of formic acid simultaneously tends to persist, causing further tissue damage and delayed regeneration of retinal function (Liu et al. 2016).
Pharmacokinetics of methanol in humans
It is known that methanol, compared to its metabolites, has much lower toxic properties toward animal cells (Dorokhov et al. 2015), and a low concentration of methanol (0.12–3.86 µg/mL) can be detected in the plasma of healthy people without optic neuropathy (Dorokhov et al. 2015; Hayasaka et al. 2000). After ingestion, methanol is easily distributed in the body—its presence in the blood, urine, cerebrospinal fluid (CSF), as well as in the vitreous and aqueous humor during a post-mortem examination of poisoned victims was detected (Benton and Calhoun 1953). Due to its rapid absorption by the oral mucosa and further parts of the gastrointestinal tract, methanol reaches its maximum concentration in the serum after 30–90 min (Abrishami et al. 2011; Liu et al. 2016). Then, methanol undergoes slow liver metabolism to its toxic metabolites: formaldehyde, the intermediate compound with a serum half-life of about 1 min, and the more stable, and toxic formic acid. It can be excreted in the urine or become decomposed into water and carbon dioxide via the tetrahydrofolate-dependent pathway (Fig. 1). These reactions are catalyzed by specific enzymes—alcohol dehydrogenase 1b (ADH1b), aldehyde dehydrogenase (ALDH), and N-10-formyltetrahydrofolate dehydrogenase (10-CHO-THF), respectively (González-Quevedo et al. 2002; Liu et al. 2016). There are two more specific pathways of exogenous methanol metabolism, the first one involving monooxygenase cytochrome P450 Family 2 Subfamily E Member 1 (CYP2E1), the activity of which is particularly expressed at high methanol concentrations in tissues, while the second pathway involves the enzyme catalase-H2O2 (Dorokhov et al. 2015). The last two pathways are responsible for 9% and 1% of methanol metabolism in the human body, respectively; however, it is postulated that their role is dominant within the central nervous system (CNS) (Dorokhov et al. 2015). Compared to ethanol—a similar compound—methanol is eliminated from the body about 6.5 times slower, which facilitates its accumulation in the body (Ingemansson 1984). According to the results of current studies, 70–97% of methanol is excreted from the body after metabolism, while only a small percentage is excreted unchanged in the exhaled air and with the urine (Dorokhov et al. 2015).
Metabolism of methanol and its metabolites in the human body. Abbreviations: 10-CHO-THF 10-formyltetrahydrofolate, ADH alcohol dehydrogenase, ALDH aldehyde dehydrogenase, ALDH1A1 aldehyde dehydrogenase 1 family, member 1, ALDH2 mitochondrial aldehyde dehydrogenase, CYP2E1 cytochrome P450 Family 2 Subfamily E Member 1, NAD nicotinamide adenine dinucleotide, NADH reduced form of nicotinamide adenine dinucleotide, NADPH nicotinamide adenine dinucleotide phosphate
Systemic manifestation of methanol intoxication in humans
The classic triad of systemic symptoms occurring in the course of methanol includes CNS depression, metabolic disorders manifested mainly as systemic metabolic acidosis, and visual disturbances (Gabay et al. 2018; Sadun 1998). As a consequence of the slow metabolism of methanol in the liver, and to a lesser extent in the blood, the symptoms of poisoning develop gradually depending on the increasing concentration of toxic metabolites in the organism (Yoo et al. 2016). Non-specific gastrointestinal symptoms including abdominal pain, nausea, vomiting, as well as other symptoms such as headache, general weakness, shortness of breath, and slight disturbances in the CNS function may occur as early as four hours after intoxication and may initially be misdiagnosed as ethanol poisoning (Grzybowski et al. 2015; Klein et al. 2017; Yang et al. 2005). Then, there is an asymptomatic period of about 10–12 h (Dorokhov et al. 2015). Usually, severe nervous system dysfunction is noticed 12–24 h after exposure to methanol (Seme et al. 2001), while visual symptoms usually appear within 12–48 h and occur in about 50% of cases (Grzybowski et al. 2015; Klein et al. 2017; Seme et al. 2001). In severe poisoning, disturbances of consciousness, memory loss, parkinsonism, severe cardiovascular symptoms, renal failure, rhabdomyolysis, coma, convulsions, and, consequently, death may occur (Choi et al. 2017; Klein et al. 2017; Yieh and Chou 2002). Lethal dose of methanol after oral ingestion is estimated to be 1.2 mL/kg (Noor et al. 2020); however, it has been shown that if the appropriate management is implemented promptly, poisoning with a much higher dose of nearly 7 mL/kg (920 mg/dL) can be successively treated (Martens et al. 1982).
Methanol-induced optic neuropathy (Me-ION)
Pathophysiology of Me-ION
Toxic exposure to methanol can result in damage to the optic nerve and to both the outer and inner retinal layers, while damage to the latter being generally more pronounced. Additionally, further parts of the visual system such as chiasm and optic tracts may also be affected (Grzybowski et al. 2015). In humans, even slight chronic exposure to low levels of methanol (0.87–1.0%) associated with severe malnutrition, especially in conjunction with a deficiency of cobalamin (vitamin B12) and folic acid—vitamins involved in the metabolism of methanol and its metabolites, can have dramatic consequences and result in the development of optic neuropathy. That was postulated in the leading hypothesis explaining the causes of the outbreak of the Cuban epidemic optical neuropathy (CEON) in 1992–1994, which affected 50,000 individuals, nearly 0.5% of the entire Cuban population (González-Quevedo et al. 2018; Hedges et al. 1997; Sadun 1998).
Previous human and animal studies have shown that systemic exposure to blood formic acid levels above 7 mM persist for more than 1 day may lead to long-term adverse visual effects (Eells et al. 1996). Importantly, animal studies have also shown that the distribution of toxic metabolites of methanol is characterized by varying intensity within the tissues. In rats, after 60 h after intoxication lasting 48 h with both low (4–6 mM) and high concentrations of methanol (8–15 mM), the highest concentration of formic acid was detected in the vitreous, retina, and blood, respectively. It is worth emphasizing that the concentration of formate in the optic nerve was five times lower than in the retina (Eells et al. 1996). While, in post-mortem human studies in methanol-poisoning victims, methanol accumulation in the vitreous was higher than in the aqueous humor (Benton and Calhoun 1953). Rodent model experiments also revealed that the distribution of methanol to tissues was independent of the degree of methanol exposure and was similar in rats intoxicated with both low (4–6 nM) and high (8–15 nM) concentrations (Eells et al. 1996).
Molecular background of functional and histopathological changes in eye tissues in the course of Me-ION
The exact cause of the marked tendency to toxic damage to the optic nerve and the retina among the eye tissues by methanol and its metabolites is not fully understood; however, it is assumed to be due to the high energy dependence of the functional profile of these tissues, which is responsible for the much greater sensitivity to mitochondrial toxins such as formic acid. It is postulated that the main phenomenon determining the occurrence of pathological changes in the eye tissues in the course of methanol intoxication is mitochondrial dysfunction secondary to the binding of formic acid to the terminal enzyme of the respiratory chain—cytochrome c oxidase, or more precisely to its ferric heme iron (Dorokhov et al. 2015). This results in inhibition of the oxidative phosphorylation process with consequent tissue hypoxia and an increase in the production of reactive oxygen species (ROS) induced by a decrease in pH—these highly reactive molecules have the ability to damage key intracellular compounds including lipids and deoxyribonucleic acid (DNA) (El-Din et al. 2011). Inhibition of oxidative phosphorylation along with a decrease in pH results in histotoxic hypoxia and tissue damage (Fig. 2) (Masoud et al. 2016). In addition, recent investigations suggest that the inflammatory background also plays an important role in the development of pathophysiological changes in the eye tissues caused by the methanol and its metabolites (Ahiskali et al. 2019; Taşlı et al. 2018).
Mechanisms responsible for the molecular background of structural and functional changes in eye tissues in the course of methanol intoxication. Abbreviations: 5-HT serotonin, 5-HT2A serotonin 2A receptor, 5-HT2C serotonin 2C receptor, Asp aspartame, ATP adenosine triphosphate, GSH glutathione, IL-1β interleukin 1β, NF-ĸβ nuclear factor-kappa B, MDA malondialdehyde, Me-ION methanol-induced optic neuropathy, MPO myeloperoxidase, OSI oxidative stress index, ROS reactive oxygen species, SOD superoxide dismutase, TAS total anti-oxidant status, TNF-α tumor necrosis factor-alpha, TOS total oxidant status.
The influence of methanol on mitochondrial function and oxidative stress parameters in the retina and optic nerve
In vitro studies with the use of formate showed that inhibition of ATP production in cultures of photoreceptor cells (661 W) and RPE (ARPE-19) increases with a decrease in blood pH; however, damage to photoreceptor mitochondria has also been found under neutral pH conditions after adding the sodium formate solution—these findings indicating the ability of formate to act as a mitochondrial toxin regardless of the pH value (Treichel et al. 2003). Animal studies based on a rat model showed the ability of retinal tissues to regain the balance of the mitochondrial energy function, but not anti-oxidant system after methanol poisoning—72 h after cessation of methanol intoxication; initially, reduced ATP and adenosine diphosphate (ADP) and increased adenosine monophosphate (AMP) concentrations did not differ significantly from the values observed in a control group; while the serum level of glutathione (GSH), a key endogenous molecule that determines the proper functioning of anti-oxidant mechanisms, was significantly decreased both during intoxication and after the 72 h observation period (Seme et al. 2001). The disturbance in the production of key molecules for cellular energy balance as one of the major mechanisms of tissue damage after methanol exposure is also supported by the results of the studies by Chen et al. where a significant reduction in the expression of ATP5A, an ATP synthase enzyme responsible for the production of ATP from ADP, was demonstrated in the retinal tissue exposed to methanol (Chen et al. 2012). Subsequent studies also showed changes in the expression of anti-oxidant defense components, as well as oxidative stress parameters. In the retinal tissue of rabbits exposed to methanol, a marked decrease in GSH concentration already after the first day reaching 60% of the initial value after 7 days along with a 70% increase in catalase serum level was found. In the same study, a change in the conformation of the rhodopsin alpha-helix, as well as the angle between rhodopsin and the cell membrane was observed, which as it can be assumed was the effect of the destructive effect of ROS on the lipid membranes structure (El-Din et al. 2011). The results of studies analyzing the effect of methanol intoxication on the levels of oxidative stress parameters in the optic nerve tissue in rats showed a decreased concentration of GSH, as well as superoxide dismutase (SOD), with a simultaneous increase in the level of myeloperoxidase (MPO) and malondialdehyde (MDA) and in the value of the index of total oxidative status (TOS), as well as the oxidative stress index (OSI), while the value of the total anti-oxidant status (TAS) was markedly reduced (Ahiskali et al. 2019; Icel et al. 2020; Taşlı et al. 2018). Interestingly, it has also been shown that intravenous ATP administration significantly reduces the negative influence of oxidative stress exerted by methanol and its metabolites on optic nerve tissue (Icel et al. 2020). To better elucidate the mechanisms responsible for the dramatic effect of methanol on mitochondrial function, Sadun developed the “mitochondrial catastrophe theory” presenting molecular changes secondary to mitochondrial damage as a vicious circle—according to this hypothesis, the decrease in the amount of ATP produced secondary to mitochondrial dysfunction results in insufficient supply of the Na + /K + ATPase pump with energy substrate, which in turn determines disruption of the axoplasmic transport of cellular organelles, including mitochondria along the axon, which leads to a decrease in the amount of available ATP at the target site (Sadun 1998).
Methanol metabolism in retinal neurons: the role of peroxisomes in its retinotoxicity
Peroxisomes are small organelles that are especially abundant in the brain, liver, and adipose tissue. Their main functions include participation in α- and β-oxidation of fatty acids, lipid synthesis, and both the production and reduction of ROS (Daniele et al. 2019). It is presumed that the peroxisomes, especially abundant in Muller cells and RPE, due to the enzymes they contain: catalase and aldehyde dehydrogenase, may be involved in the local metabolism of methanol, which determines the formation of formaldehyde and formic acid (Garner et al. 1995; Glorieux and Calderon, 2017; Hayasaka et al. 2001; Icel et al. 2020; Sharma et al. 1999). This theory is supported by the results of studies in which the formate level in the retinal tissue was five times lower after systemic administration of formate compared to that measured after systemic administration of methanol at the corresponding dose (Garner et al. 1995). Therefore, this phenomenon may be an additional factor contributing to retinal damage.
Formaldehyde toxicity
Although the most pronounced ocular tissue toxicity following methanol intoxication is attributed to formate, the role of formaldehyde toxicity should not be excluded. As mentioned, aldehyde may be produced locally in the retina as a result of methanol metabolism. Similarly, current animal studies have demonstrated the ability to metabolize methanol to formaldehyde within the CNS in primates after intracerebroventricular injection of a single dose of 200 µL of 5% methanol (Zhai et al. 2016). An experiment on the rat model showed abnormalities found in fundoscopy after 7 days, as well as 1 month after the injection of 0.1 and 1.0% formaldehyde solutions. Moreover, in the histopathological examination, pathological changes such as disorganization of the outer nuclear layer (ONL) and ganglion cell layer (GCL) structures were found after the injection of 0.1% formaldehyde. While the injection of 1% solution caused diffused disturbances in the structure of the retina. The administration of both mentioned concentrations of formaldehyde caused vacuolization of the optic nerve (Hayasaka et al. 2001).
The role of serotonin (5-HT) and aspartame (Asp) in the course of Me-ION
It has been proven that apart from disturbing the process of energy transformations and the destructive influence of ROS, other mechanisms may also be responsible for the negative influence of methanol on the optic nerve and the retina. Increased concentrations of aspartame (Asp) in the optic nerve and serotonin (5-HT) in the retina were observed in rats chronically intoxicated with low doses of methanol (2 g/kg for 14 days) (González-Quevedo et al. 2002). Interestingly, elevated levels of Asp and glutamate (Glu) were also detected in CSF samples of CEON patients in whom chronic exposure to low levels of methanol along with folate deficiency was the most likely pathophysiological background (González-Quevedo et al. 2002). It is believed that the retinotoxicity of Asp is based on the mechanism of increased influx of calcium ions into cells due to the stimulation of N-methyl-D-aspartate (NMDA) receptors, the richness of which has been demonstrated within the retinal ganglion cell (RGC) layer from where the optic nerve axons originate (Shen et al. 2006). On the other hand, it is postulated that an increase in serotonin (5-HT) concentration results in the stimulation of 5-HT2A and 5-HT2C receptors in retinal neurons with a subsequent increase in intracellular calcium ion concentration, which, due to the disturbance of intracellular calcium homeostasis, promotes activation of signaling pathways responsible for retinal neurons death (González-Quevedo et al. 2002; Icel et al. 2020; Masson 2019).
Inflammatory response secondary to methanol intoxication
Previous animal studies have shown that methanol intoxication can affect the function of the neuroimmune system and induce both specific and non-specific systemic immune responses, probably mainly by increasing oxidative stress and secondary changes in corticosterone levels resulting from dysfunction of the hypothalamic–pituitary axis (Moral et al. 2015; Parthasarathy et al. 2006, 2007). Examination of the optic nerve tissue of rats treated with the combination of methotrexate (MTX) and methanol showed higher expression of the final DNA degeneration product—8-hydroxy-2'-deoxyguanosine (8-OHdG), as well as molecules involved in the inflammatory response such as tumor necrosis factor-alpha (TNF-α), nuclear factor-kappa β (NF-kβ), and interleukin 1 beta (IL-1β), which suggests that the inflammatory background also plays an important role in the development of pathophysiological changes in the eye tissues caused by the methanol and its metabolites (Taşlı et al. 2018).
Retinal proteins: changes in their expression secondary to methanol exposure
The results of current research on animal models indicate the possibility of much greater complexity of mechanisms determining cytotoxicity associated with methanol consumption. The toxic effect of methanol on the optic nerve and the retina may occur through the influence on the expression of key cytoskeleton proteins—actins and tubulins (beta-actin, beta-tubulin) conditioning the maintenance of the proper structure of cells, as well as cell signaling and regulating the process of apoptosis. In addition, changes in the expression of enzymes from the group of alcohol dehydrogenases: alcohol dehydrogenase (ADH), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), long-chain acetyl-CoA dehydrogenase (VLCAD), family 5 member aldehyde dehydrogenase A1 (ALDH5A1), dehydrogenase 2 monophosphate-5' (IMPDH2), as well as proteins from the crystallin group and heat shock proteins (HSP) were also observed (Chen et al. 2012; Huang et al. 2011).
Histopathological changes in eye tissues in the course of Me-ION
The number of histological studies of methanol toxicity to ocular tissues in humans is low, and the available results are limited to post-mortem studies and do not allow to fully assess which changes are a direct result of the action of methanol and its metabolites and which are post-mortem artifacts (Murray et al. 1991). Thus, a lot of valuable information was obtained using animal models in which mainly rodents were intoxicated. However, due to the much more efficient oxidation of formic acid in non-primates, due to the high activity of 10-CHO-THF, which in humans only reaches 26% of that observed in rodents, models using non-primates did not fully allow simulate the human conditions (Chen et al. 2012). Hence, to obtain a similar sensitivity to the toxicity of methanol and its metabolites as observed in humans, the animals were exposed to nitric oxide (NO), which inhibited the activity of methionine synthase (MS), and resulted in a secondary tetrahydrofolate deficiency, as well as inhibition of the activity of 10-CHO-THF—the decreased activities of these two enzymes lead to impaired metabolism of formate and its accumulation in the body (Chen et al. 2012). Another frequently used method is the introduction of a folate-deficient diet or the pharmacological induction of a folate deficiency by administering MTX (Eells et al. 1996).
Animal histopathological studies
Chen et al. showed that in rats after intoxication with methanol, histopathological changes manifested mainly as mitochondrial damage are first found within the outer layers of the retina, starting from the photoreceptor layer, and spread further into the retinal inner nuclear layer (INL) (Chen et al. 2013). These observations are supported by the results of in vitro studies on the ocular cells where a decrease in ATP concentration after the administration of formate was detected earlier within the photoreceptor cell line (661 W) compared to the RPE cell line (ARPE-19). Moreover, also the histopathological changes were more pronounced in the 661 W cells, and the decrease in ATP concentration reflecting the disturbance of mitochondrial function at the examined time points correlated with higher levels of the cytotoxicity marker—lactate dehydrogenase (LDH) (Treichel et al. 2003). It is also supposed that the high content of polyunsaturated fatty acids (PUFAs), especially arachidonic acid (AA; 20:4n 6), in the photoreceptor layer may contribute to the damage of this layer by ROS in the lipid peroxidation process, mainly by its final product, malondialdehyde (MDA) (Ahiskali et al. 2019; Seme et al. 2001; Su et al. 2019). These findings suggest a greater structural and functional sensitivity of photoreceptors to formate-mediated toxic damage and indicate this retinal layer as the primary target of methanol-induced toxicity. Formic acid can penetrate the optic nerve from the CSF, as well as through the choroidal circulation, where it reaches a particularly high concentration in the retrolaminar region (Kavet and Nauss 1990). Histopathological analysis of the retinas and optic nerves of rats intoxicated with methanol showed pathological changes within the optic nerve constituting from the axons of retinal ganglion cells (RGCs), where pathological changes mainly concerned the prelaminar region and were expressed as axonal vacuolation and edema of the oligodendroglia and astrocytes manifested as myelin sheath damage (Eells et al. 1981, 2000; Galvez-Ruiz et al. 2015; Hayreh 1989). Vacuolisation of the axons of the laminar and post-laminar regions of the optic nerve head was also observed (Moschos et al. 2013; Rotenstreich et al. 1997). Examination of the optic nerves of Rhesus monkeys intoxicated with methanol revealed abnormalities mainly in the intraocular part of the optic nerve (Baumbach et al. 1977). In addition, axonal myelin sheath shrinkage was observed, causing axonal compression and honeycomb pattern in a histopathological examination—it is assumed that these findings result from the greater susceptibility of white matter composed of oligodendroglial cells to formate-mediated toxicity due to the lower content of cytochrome oxidase c compared to gray matter (Baumbach et al. 1977; Kavet and Nauss 1990). Thus, it is believed that the disruption of axoplasmic flow secondary to both ATP deficiency and mechanical axon compression by the contracted myelin sheaths caused by histotoxic edema of glial cells leads to stasis of the axoplasmic flow and plays a significant role in optic nerve damage (Baumbach et al. 1977). Ultrastructural changes at the axonal level observed by electron microscopy in addition to early degenerative changes included focal edema, axonal debris accumulation, fluid pocket formation, neurofibril breakdown, and axon-associated glial edema, which in some cases showed signs of gliosis. Mitochondria were characterized by a more elliptical shape, as well as larger and denser cristae or their disappearance (Sadun 1998; Seme et al. 2001). At the ultrastructural level, abnormalities in each of the layers of the retina can be observed. Evaluation of the methanol-exposed rats’ retinas revealed that within the RPE layer, vacuolization was dominant. Damage to the photoreceptors within the inner segments, where disorganization and swelling can be found, while the outer segment may become fragmented, and damage to the photoreceptor nuclei may also be present. Other studies have shown that vacuolization within the junction of the inner and outer segments of photoreceptors is also a distinctive finding (Eells et al. 1996; Rasheed et al. 2017; Seme et al. 1999, 2001). Typically, the mitochondria of the photoreceptor inner segments were damaged—swollen with enlarged or absent cristae; moreover, the number of these cell organelles may be reduced (Eells et al. 2003; Rasheed et al. 2017; Seme et al. 2001). In the outer nuclear layer (ONL), pathological changes manifested as edema and an increase in the space between the photoreceptor nuclei were noted; some of the nuclei were characterized by reduced size and apoptotic changes. Outer limiting membrane (OLM) may be interrupted or atrophic, while the outer plexiform layer (OPL) may have increased thickness (Rasheed et al. 2017; Zarenezehad et al. 2013). Irregular inner nuclear layer (INL) structure may also be present with apparent swelling and fragmentation of the nuclei. Edema may also occur within the inner plexiform layer (IPL); while RGCs may present apoptotic changes and vacuolation, and their number may be reduced (Rasheed et al. 2017).
Human histopathological studies
The most characteristic changes reported by Sharpe et al. in four patients who died due to methanol poisoning were demyelinating changes in the retrolaminar region and swelling of the optic disc, appearing 2 days after intoxication, probably as a result of axoplasmic stasis (Sharpe et al. 1982). Damage to the myelin sheath in the retrolaminar region of the optic nerve was also noted by Naeser (Naeser 1988). In this case, retinal abnormalities including RGCs enlargement, moderate disturbances of the INL and ONL structure, as well as granulocytic infiltration of IPL were also observed (Naeser 1988). Postmortem examination of a patient who died in the course of CEON showed scarcity with concomitant chromatolysis of the RGCs nuclei with no changes in the photoreceptors and the peripheral retina. Additionally, reduced thickness of retinal nerve fiber layer (RNFL) within papillomacular bundle (PMB) was revealed. Interestingly, the reduced RNFL thickness in the PMB region was also observed in optical coherence tomography (OCT) examinations, and is presumed due to the increased sensitivity of small diameter mitochondria-rich nerve fibers to toxic formate damage (Nurieva et al. 2018). Histological analysis of six eyeballs enucleated for 6–18 h in victims of accidental methanol poisoning revealed degenerative changes in the photoreceptor layer and RGCs, as well as swelling of the optic disc and the peripapillary retina (Benton and Calhoun 1953).
Assessment and diagnosis of Me-ION
Diagnosis in the case of suspicion of Me-ION is based on collecting a detailed medical history, especially focused on the possibility of consumption or contact with chemical substances containing methanol, laboratory tests, as well as detailed ophthalmological examination (Sharma et al. 2012).
Laboratory tests
Laboratory tests revealing severe metabolic acidosis with the accompanying anion gap and plasma osmolality gap without any other clear cause should lead to the suspicion of methanol poisoning (Noor et al. 2020). Arterial blood pH has been shown to correlate with serum formate levels negatively, and a pH value below 7.2 indicates severe intoxication (Sharma et al. 2012). The concentration of methanol in the peripheral blood measured using liquid or gas chromatography exceeding 20 mg/dl allows to confirm the diagnosis of methanol poisoning (Sharma et al. 2012); however, due to the high cost, time, and low availability of this method in smaller centers, it may not always be used in the emergency conditions (Kraut 2016). Moreover, despite the usefulness of determining the serum concentration of methanol in the diagnosis of poisoning, it should be emphasized that this parameter does not correlate with the degree of intoxication of the organism and for this purpose, the assessment of arterial blood pH is much more valuable (Ma et al. 2019).
Visual symptoms of Me-ION
Me-ION is usually bilateral, symmetrical, and in most cases presents a severe, rapidly progressive course (Sharma et al. 2012). During the presentation, patients may report eye pain located most often behind the eyeball, photophobia, and visual disturbances, which can manifest as either a slight reduction in visual acuity, up to the loss of vision, including a lack of light perception (Kraut 2016; Seme et al. 2001). On physical examination, eye redness and decreased pupil diameter or unresponsiveness of the pupils to light may be identified (Kraut 2016). Other symptoms, including the impaired contrast sensitivity, dyschromatopsia, blurred vision, diplopia, central or ceocentral scotoma with or without peripheral visual field defect, and more rarely, saccadic eye movements, as well as visual hallucinations may also be present (Ingemansson 1984; Sadun et al. 1994; Sharma et al. 1999). Importantly, in the case of chronic exposure to methanol leading to gradual intoxication, systemic and ocular symptoms may be initially limited and then recur with a stronger manifestation until a sudden crisis (Ma et al. 2019).
Eye findings in patients with Me-ION
Slit-lamp examination of the anterior segment of the eye usually does not reveal significant abnormalities, although in some cases, fixed and dilated pupils with no response to light stimulation may be observed, this sign reflecting an unfavorable prognosis of final visual acuity and survival (Barceloux et al., 2002; Benton and Calhoun 1953). Intraocular pressure (IOP) is usually within the normal range (Klein et al. 2017; Sharma et al. 2012; Yang et al. 2005; Yoo et al. 2016). Analysis of the results of an ophthalmological examination after the outbreak of mass methanol poisoning in Atlanta showed the presence of fundus lesions in 87% of patients with initial vision loss and in nearly 100% of patients in whom continued follow-up examination showed a persistent decrease in visual acuity (Benton and Calhoun 1953). A fundus examination usually reveals bilateral hyperemia and edema of the optic disc, manifesting 6–24 h after methanol intoxication, with or without accompanying edema of the peripapillary RNFL, which may precede the onset of visual disturbances (Sharma et al. 2012; Yang et al. 2005; Yieh and Chou 2002). It is known that retinal edema is frequently observed along the major retinal vascular arches and may persist for 10–60 days (Benton and Calhoun 1953; Ingemansson 1984), then, after the resolution, transforms into retinal atrophy (Ingemansson 1984). In some cases, the characteristic wedge-shaped defects of RNFL may be present (Sadun et al. 1994). Macular edema along with the disappearance of macular reflex has also been reported (Ruedemann 1962). Changes in the optic disc appearance evolve with the course of intoxication from normal to swollen with dilated peripapillary and tortuous retinal vessels in acute, early phase (Cursiefen and Bergua 2002) to excavated and pale, in some cases glaucomatous-like with atrophy of the neuroretinal rim during the chronic phase, which is a sign of progressive degenerative changes (Grzybowski et al. 2015; Hayasaka et al. 2001; Moschos et al. 2013; Shin and Uhm 2011). These abnormalities are most likely developing based on acute RGC damage and progressive demyelination of nerve fibers, usually 30–60 days after intoxication (Benton and Calhoun 1953; Hayasaka et al. 2001; Yang et al. 2005). The atrophy of the optic disc may be more pronounced in the temporal segments (Nurieva et al. 2018). In sites of primary retinal edema, weakness and sheating of arterioles may be observed as a result of post-inflammatory changes (Dethlefs and Naraqi 1978).
Imaging tests in the diagnostic process of Me-ION
The use of imaging tests plays an important role both at the initial stage of the diagnostic process as well as in monitoring the ophthalmic condition of patients affected by Me-ION. In the acute phase, OCT of the peripapillary region may show localized RNFL edema accompanied by the presence of intraretinal fluid (Fujihara et al. 2006), while in the late stage, OCT of the optic discs exhibits significant RNFL thinning that may progress up to 4 years after intoxication (Abrishami et al. 2011; Koehrer et al. 2011; Moschos et al. 2013; Nurieva et al. 2018; Shin and Uhm 2011). Macular OCT changes are characterized by a variable presentation—starting with no significant structural changes in the acute phase (Abrishami et al. 2011; Fujihara et al. 2006) by diffuse retinal thinning in the macular region observed 2 months after intoxication, to macular edema leading to flattening of the fovea with the presence of INL cysts observed 3 months after intoxication as a result of RGC loss (Fujihara et al. 2006). Interestingly, despite the particular susceptibility to damage to the photoreceptor layer observed in histopathological studies, the morphology of this layer in the reported OCT scans was normal (Koehrer et al. 2011). Fluorescein angiography (FA) initially reveals hyperfluorescence with late leakage in the peripapillary area; in addition, when retinal edema is present, this region may be characterized by late hypofluorescence (Fujihara et al. 2006); otherwise, multiple focal RPE detachments as an effect of intraretinal fluid accumulation have been observed (Ranjan et al. 2014). The classic finding in magnetic resonance imaging (MRI) is basal ganglion degeneration, typically with bilateral hemorrhagic or non-hemorrhagic putamen necrosis and edema or non-specific demyelinating lesions located in the deep white matter; whereas in toxic optic TONs of a different cause, these changes are usually not present (Grzybowski et al. 2015; Ma et al. 2019; Yang et al. 2005). Ma et al. described the bilateral enhancement of the signal of the optic nerves within the orbits and in the canal part as a frequent find in patients after methanol poisoning (Ma et al. 2019). At the same time, increased uptake of contrast in retrobulbar segments of optic nerves followed by slow diffusion of contrast has also been reported (Tanrivermis Sayit et al. 2016).
Electrophysiological tests in the diagnosis of Me-ION
The importance of the application of electrophysiological tests such as electroretinography (ERG) and visual evoked potentials (VEPs) in the diagnostic process of Me-ION is indisputable. According to the results of animal studies, the abnormalities found in electrophysiological tests may precede the structural changes in the retina and optic nerve observed in fundus examination or imaging tests (Eells et al.1996; Seme et al. 1999).
Visual evoked potentials (VEPs)
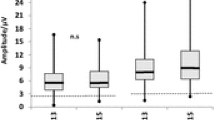
The results of VEPs usually show normal or less frequently prolonged latency, as well as abnormalities in waveform including weak or persistent no fixed waveforms, while the P100 amplitude in most cases is reduced (Grzybowski et al. 2015; Koehrer et al. 2011; Moschos et al. 2013). Interesting observations were made by McKellar et al., who observed a reduction in the P2 amplitude without significant changes in its latency, which is in contrast to the typical changes usually seen in optic neuropathies. Follow-up examination showed that the above-mentioned changes resolved 28 days after intoxication (McKellar et al. 1997). Another reported patient had mf-VEP abnormalities in area 0 (Moschos et al. 2013).
Electroretinography (ERG)
Animal studies using ERG suggest that photoreceptors exhibit heterogeneous sensitivity to the toxic effects of methanol and its metabolites (Chen et al. 2013; Seme et al. 1999, 2001). In rats intoxicated with methanol, a partial recovery of the rod response to 15 Hz/510 nm stimulation was observed, while the UV-cone-mediated function showed no signs of regeneration in the ERG over the 72 h observation period (Seme et al. 1999). Similarly, the experiment of Chen et al. revealed the more pronounced cone damage in the F-ERG recording in rats after a 7 day period of methanol intoxication (Chen et al. 2013). On the other hand, Liu et al. showed no improvement in both scotopic and photopic recordings of ERG in rats performed on the third and seventh day after intoxication (Liu et al. 2016). In case reports involving human ERG recordings, a and b wave amplitude reduction is typically seen after both acute and chronic methanol exposure (Ingemansson 1984; McKellar et al. 1997; Ruedemann 1962), which proves abnormalities in the process of photoreceptor hyperpolarization, as well as depolarization disorders of Muller glial cells with disturbances in transmission between photoreceptors, bipolar cells, and RGCs, respectively (Eells et al. 1996; McKellar et al. 1997). Moreover, in severe cases, ERG may not be registered even several months after poisoning (Fujihara et al. 2006); interestingly, in the case described by McKellar et al. despite the initially observed abnormalities in the F-ERG, the scotopic and cone-mediated responses returned to normal on the 14th and 21st days after the poisoning, respectively (McKellar et al. 1997), which proves the ability to recovery photoreceptor function in humans, similarly as it was observed in animal studies. In rats subjected to long-term exposure to low concentrations (4–6 nM) of methanol, a significant reduction of the a and b amplitude in ERG was observed despite the absence of systemic markers of intoxication such as metabolic acidosis and histopathological changes in eye tissues (Eells et al. 1996). Importantly, in other human and animal studies, abnormalities in ERG were recorded at methanol concentrations that did not cause metabolic acidosis, disturbances in pupil reactivity, pathological fundus changes, and VEP abnormalities (Eells et al. 1996); these results show high specificity and greater sensitivity of ERG compared to the use of VEP in the diagnosis of patients with a suspicion of methanol poisoning (Plaziac et al. 2003). Moreover, the results of studies evaluating changes in ERG in intoxicated animals showed that the reduction in the amplitude of the b wave occurred faster and was more pronounced compared to the a wave, with the amplitude reduction of both waves being proportional to the increasing accumulation of formic acid in the serum (Eells et al. 1996). In animals exposed to lower concentrations of methanol (4–6 nM), deviations in ERG were recorded after 60 h, compared to 24 h in animals intoxicated with high concentrations of methanol (7–15 nM); this difference demonstrating a time-dependent relationship between the rate of intoxication and the occurrence of retinal dysfunction (Eells et al. 1996). Consistent results have also been obtained in other animal studies evaluating ERG after methanol exposure. In methanol-intoxicated rabbits, 1-week observation showed a linear decrease in the amplitude of the b wave reaching 35% in comparison to the initial value; amplitude of the a wave showed a clear tendency to stabilize (El-Din et al. 2011). In rats exposed on methanol at a dose of 1.5–4 g/kg (Garner et al. 1995; Lee et al. 1994; Murray et al. 1991; Plaziac et al. 2003) and inhaled methanol at a dose of 2000 ppm (Lee et al. 1994) also marked susceptibility of the b wave to the influence of methanol compared to the a wave was observed (Garner et al. 1995; Lee et al. 1994; Murray et al. 1991; Plaziac et al. 2003). In primates, exposure to methanol at a dose of 6 g/kg caused a reduction in the amplitude of the b wave and attenuation of the a wave (Potts et al. 1955); while in monkeys exposed to a lower dose of methanol, reaching 2–5 g/kg, no changes in the ERG were found (Blomstrand and Ingemannson 1984).
Oscillatory potentials (OPs)
The ERG oscillatory potentials (OPs), also called low-voltage high-frequency oscillations, are localized on the ascending arm of the B wave and reflect low-amplitude impulses generated mainly by amacrine, bipolar, and interplexiform cells (Gauvin et al. 2016). Analysis of ERGs in methanol-intoxicated rats showed a reduction in summed amplitudes of wavelets (SOAP), as well as elapsed time (ET) delay which indicates damage to the receptor function of the retina. Similarly, the length of the interpeak interval (IPI) is 1–4, especially the IPI 2 increased with the length of the exposure period. Additionally, compared to non-intoxicated rats, a significant reduction in the amplitude of OS1, OS3, OS4, and OS5 was observed both after 3 and 7 days after methanol administration (Liu et al. 2016). Similarly, Plaziac et al. noted a significant reduction in OPs already 24 h after the exposure, which was maintained for the next 3 days (Plaziac et al. 2003). Interestingly, in both studies, the amplitude of OP2 was reduced to a lesser extent, while the most marked reduction concerned OP3 and OP4 (Liu et al. 2016; Plaziac et al. 2003). It is believed that short-latency OPs (OP1 and OP2) are generated mainly by cones, while rods play a major role in the formation of longer latency OPs (OP3, OP4, and OP5) (Liu et al. 2016).
Application of electrophysiological tests in the research on new methods of Me-ION therapy
Seme et al. hypothesized that the lower sensitivity of the cone-mediated 15 Hz/510 nm response to methanol-induced damage demonstrated in the previous studies (Seme et al. 1999), and their impaired ability to recover after intoxication, suggests that they are less susceptible than rods to acute methanol damage; however, after marked disruption, cones do not show a tendency to regenerate (Seme et al. 2001). Current in vitro studies investigating the role of Muller cells as stem cells in the treatment of retinal injuries may shed new light on partial return of rod function after methanol exposure observed by Seme et al. (Seme et al. 2001). It has been shown that Muller cells are activated secondary to retinal damage and have the ability to migrate and proliferate at the site of injury mainly due to the activity of bioactive sphingolipid compounds (Hamon et al. 2016; Lenkowski and Raymond 2014; Vera et al. 2021). In vitro studies using human Muller cell lines have shown their ability to restore the function of both rods and RGCs (Hamon et al. 2016; Jayaram et al. 2014; Singhal et al. 2012). Despite the lack of in vivo human results, this hypothesis may explain this interesting phenomenon, and methods of regeneration of RGCs and photoreceptors may in the future constitute a new therapeutic option for patients affected by Me-ION.
Treatment of Me-ION
Immediate initiation of treatment in Me-ION plays a key role in the prognosis of visual function after survival (Tanrivermis Sayit et al. 2016). Systemic treatment of methanol poisoning should be directed toward three main goals: first, limiting metabolic acidosis, second, inhibiting hepatic metabolism of methanol to its toxic metabolites, and third, facilitating the elimination of these compounds from the body (Barceloux et al. 2002; Sharma et al. 2012). These three steps in the treatment of methanol intoxication allow minimizing the toxic effects of methanol on tissues, including the eye tissues.
In the correction of metabolic acidosis, hemodialysis and intravenous bicarbonate administration are mainly used (Sharma et al. 2012). Hemodialysis allows for the rapid removal of methanol and formic acid from the body (Kraut 2016), while the increase in pH secondary to the use of bicarbonates reduces the entry of formic acid into the cells by increasing the ionization of methanol, and thus alleviating tissue damage (Garner et al. 1995; Tasli et al. 2018). The oxidation of methanol by alcohol dehydrogenase (ADH) is a key stage leading to the formation of its toxic metabolites in the body—hence, the first-line antidotes used in methanol poisoning are the competitive inhibitors of alcohol dehydrogenase—ethanol and fomepizole, also known as 4-methylpyrazole, preventing methanol metabolism to its toxic metabolites (Noor et al. 2020); their use allows to increase the ratio of unchanged methanol excreted from the body to metabolized methanol (Rasheed et al. 2017; Yieh and Chou 2002). Fomepizole has 500–1000 times stronger affinity for alcohol dehydrogenase compared to ethanol (Kraut 2016; Sharma et al. 2012); however, due to the much higher cost of fomepizole therapy, as well as a similar side effect profile and mortality rate, ethanol is a much more widely used antidote, especially in developing countries (Kraut 2016; Noor et al. 2020). It has been shown that methanol is oxidized in the body by alcohol dehydrogenase about ten times slower than ethanol (Rasheed et al. 2017), and the target serum ethanol concentration having an inhibitory effect on methanol conversion by ADH is 100–150 mg/dL (Kraut 2016). The tetrahydrofolate pathway plays a key role in the metabolism of formic acid to non-toxic metabolites—carbon dioxide and water (Abrishami et al. 2011). Thus, it is believed that the use of folinic acid may accelerate the metabolism of formic acid and, similarly to the use of hemodialysis, facilitate the elimination of methanol, and therefore shortening the time of its harmful effect on the body (Sharma et al. 2012). Dual therapy based on an ADH inhibitor and hemodialysis should be preferred when available, as this approach reduces the body’s exposure to methanol, preventing further complications, which contributes to lower treatment costs (Kraut 2016).
Glucocorticosteroids
The initial phase of methanol-induced optic nerve damage is believed to resemble optic neuritis in its pathophysiological course (Permaisuari et al. 2019). Thus, the use of glucocorticosteroids (GCs) having the ability to down-regulate the expression of pro-inflammatory cytokines and up-regulate the expression of anti-inflammatory molecules, as well as neuroprotective and anti-demyelinating properties, has been implemented in Me-ION cases (Stunkel and Van Stavern 2018).
In the treatment of Me-ION, GCs are usually administered intravenously at a daily dose of 1 g methylprednisolone over 3–4 consecutive days, followed by oral prednisolone at a dose of 1 milligram per kilogram of body weight daily (Abrishami et al. 2011; Masoud et al. 2016; Sodhi et al. 2001). Abrishami et al. presented the results of an interventional case series involving six men poisoned with methanol after consuming homemade alcoholic beverages treated with 250 mg of intravenous methylprednisolone every 6 h for 3 days. Importantly, there was no additional treatment with hemodialysis, ethanol, or vitamins. The post-treatment examination showed an improvement in the mean BCVA 0.86 ± 0.08 vs 0.33 ± 0.18 and 0.93 ± 0.1 and 0.29 ± 0.2 for OD and OS, respectively (Abrishami et al., 2011). In other case series and case reports, a beneficial effect of steroid therapy on long-term visual sequelae has also been observed (Kowalski et al. 2019; Masoud et al. 2016; Sodhi et al. 2001). A collective statistical analysis including the results of published studies indicates the effectiveness of steroid therapy leading to the improvement of vision in more than 80% of eyes; however, it is worth emphasizing that the available studies were carried out on small groups without controls, on a case series or case reports (Permaisuari et al. 2019). Reports regarding late initiation of GCs therapy—more than 6 days after intoxication—show mixed results including no effect (Fujihara et al. 2006; Koehrer et al. 2011), as well as improvement of visual acuity (Rotenstreich et al. 1997). Shukla et al. in the group of 17 patients showed no association between the time of starting treatment with steroids, which reaching from 6th to 45th day, and the final visual acuity (Shukla et al. 2006). Therefore, further studies are needed to evaluate the efficacy of GCs use in Me-ION patients fully. According to the present knowledge, their role as an additional adjunctive treatment to conventional therapy appears to be beneficial and their inclusion in Me-ION patients in the absence of general contraindications should be considered.
Erythropoietin
In recent years, the use of erythropoietin (EPO) due to its neuroprotective effect on glial cells, anti-inflammatory, anti-apoptotic, and anti-oxidant effects, as well as ability to improve the blood supply to damaged tissue has been recognized as a promising therapeutic method that can improve the visual acuity of patients suffering from different types of optic neuropathies (Pakravan et al. 2016). The beneficial effects of EPO in patients with toxic damage to the optic nerve secondary to methanol exposure are particularly presumed to be due to its ability to inhibit axon and RGC apoptosis, as well as the anti-oxidant activity exerted by an increase in the activity of two key enzymes in the anti-oxidant defense system—GSH and SOD (Pakravan et al. 2016). To date, one double-blind randomized clinical study and one case series investigating the effect of intravenous administration of EPO in Me-ION have been conducted, and both have shown promising results (Pakdel et al. 2018; Pakravan et al. 2016). Among two studies comparing the use of EPO as an adjunct to methylprednisone therapy, one revealed only transient visual acuity improvement in Me-ION patients treated with the combination of EPO and GCs. Better results in the group of patients in whom EPO have not been added were observed after follow-up period. However, in the second experiment including larger sample size, significant improvement in patients treated with EPO and GCs compared to therapy with EPO alone was noted (Pakravan et al. 2016; Zamani et al. 2018) (Table 1).
Photobiomodulation
Photobiomodulation (PB), also known as low-intensity light therapy, involves applying light with a wavelength of 630–1000 nm and a far-red to near-infrared (NIR) spectrum emitted by low-level lasers (LLLs) or light-emitting diodes (LEDs) to modulate specific cell functions (Desmet et al. 2006). Although the exact mechanism of the beneficial effect of this method has not been fully understood, it is assumed that PB improves mitochondrial function by stimulating the activity of cytochrome c oxidase c complex IV, leading to an increase in ATP synthesis (Yu-Wai-Man et al. 2014).
PB has been shown to have neuroprotective properties, promote the wound-healing process, reduce heart damage after ischemia, and may also be useful in patients with congenital (e.g., Leber's congenital optic neuropathy and autosomal dominant optic atrophy), as well as acquired toxic-mediated injury of the optic nerve and retina, including the Me-ION (Desai et al. 2013). The animal study showed a protective effect on the photoreceptors function and retinal structure after three 144 s sessions of NIR-LED light with a wavelength of 670 nm applied at 5, 25, and 50 h after intoxication with methanol (Eells et al. 2003). In this study group, after the use of high light intensity PB, a partial recovery of the function of UV-cones, M-cones, and rods was observed in rats. In addition, in the LED-treated group, no histopathological changes of the retina were shown either in light microscopy or at the ultrastructural level, while in the group without PB application, clear pathological changes were observed (Eells et al. 2003).
Anti-oxidants
In recent years, promising results of animal studies investigating the effect of using compounds with anti-oxidant properties such as taxifolin (Ahiskali et al. 2019), ATP (Icel et al. 2020), as well as rutin (Taşlı et al. 2018) have been published. The above studies showed a significant effect of these compounds on the alleviation of methanol-induced oxidative stress in the nerve tissue of rats. Another study showed the protective potential of 4-hydroxy-2,2,6,6-tetramethylpiperidinyl-1-oxyl (TEMPOL)—a chemical compound having SOD mimetic properties on RGCs exposed on methanol (Setiohadji et al. 2018). However, further research is needed to evaluate the efficacy of these antioxidants in humans fully. Additionally, in some cases, the vitamin B group (e.g., vitamin B1, B6, and B12) has been used (Rotenstreich et al. 1997); but there are no studies in which their protective potential to ocular tissues in patients with Me-ION has been investigated.
Prognosis of patients with Me-ION
Ocular prognosis
In most patients, visual disturbances usually resolve within 2–3 weeks after intoxication; however, persistent visual sequelae are estimated to affect more than 33–40% of patients (Nurieva et al. 2018). Moreover, newly emerging or initially undiagnosed ailments may be found many months after intoxication (Nurieva et al. 2018; Paasma et al. 2007). To date, the results of several studies assessing the prognosis of visual complications in patients after methanol poisoning have been published. A 3 month follow-up of 24 patients from Port Moresby in Papua New Guinea showed that the presence of metabolic acidosis on admission and the amount of consumed methanol positively correlated with the occurrence of persistent visual impairment (Dethlefs and Naraqi 1978). Sullivan-Mee and Solis hypothesized that the pupil reactivity in the initial examination may provide important prognostic information for the further visual function, and their abnormal response is associated with a worse prognosis and a greater risk of permanent visual abnormalities (Sullivan-Mee and Solis 1998). This assumption was indirectly confirmed by the results of a study evaluating the presence of long-term visual sequelae in a group of 122 individuals after methanol poisoning, where diminished pupil reactivity and abnormalities in the fundoscopy during the initial examination were observed significantly more often in patients with arterial blood pH value lower than 7.2 measured on admission, and follow-up showed less improvement and lower visual acuity after 3 months (Desai et al. 2013).
Interesting results were obtained in the studies of the population affected by Me-ION as a result of massive poisoning in the Czech Republic in 2012, including 139 cases, and more than 50 deaths (Nurieva et al. 2018; Zakharov et al. 2014). Zakharov et al. showed that despite the presence of visual symptoms in 14% of patients at discharge, the frequency of the occurrence of persistent visual sequela in the 3–8 month period of follow-up increased to 40%, and blindness affected 8% of patients (Zakharov et al. 2014). Risk factors associated with a higher probability of persistent visual sequelae were visual disturbances and coma revealed on admission, while concomitant ethanol consumption significantly reduced the likelihood of their occurrence. In addition to the analysis of the correlation between clinical parameters, the assessment of RNFL thickness using OCT and the evaluation of VEP components—P1 latency—as an indicator of the demyelination and remyelination process, as well as the N1P1 amplitude—as an indicator of RGCs damage were used to determine the prognosis in patients with Me-ION. Pathological changes in RNFL and abnormalities observed in VEP recordings were detected in a follow-up examination 3–8 months after discharge in 38% and 40% of survivors after methanol poisoning, respectively, with 28% borderline results in RNFL, and 18% borderline results in VEP (Zakharov et al. 2015). It was shown that the presence of serum ethanol on admission was associated with a lower probability of damage to the axons of the optic nerve and a higher RNFL thickness measured during the observation period. Moreover, RNFL thinning and abnormalities in VEP showed a mutual correlation, which reached statistical significance in the second, but not in the fourth year after discharge (Nurieva et al. 2018; Zakharov et al. 2015). In 22% of patients without visual symptoms at discharge, RNFL thinning and VEP abnormalities were observed after 3–8 months (Zakharov et al. 2015). P1 latency abnormalities were observed on admission in 50% of patients; however, during the surveillance period, a significant reduction in its duration was observed in cases where the initial P1 latency length corresponded to mild or moderate damage to the myelin sheath, which proves the ability to remyelinate nerve fibers after acute methanol damage (Nurieva et al. 2016). Significantly in 2 years of observation, chronic alcohol abuse was associated with worse effectiveness of the remyelination process, while beneficial factors for the regeneration of myelin were both lower serum concentrations of carbohydrate-deficient transferrin and methanol, as well as higher blood pH value during the presentation (Nurieva et al. 2016). Additionally, in older individuals, longer P1 latency during 2 year follow-up was observed, which indicates a lower efficiency of the optic nerve axon remyelination process (Nurieva et al. 2016). Moreover, a significant association between the risk of thinning RNFL, and the value of arterial blood pH measured at presentation, as well as the abnormal morphological changes in the CNS on MRI, were also found (Nurieva et al. 2016). The reduced P1 latency observed in the VEP returned to normal during the 2 year follow-up period, probably due to remyelination process of the nerve fibers; therefore, the initially observed relationship between P1 latency extension and RNFL thinning became insignificant at the end of the observation period (Nurieva et al. 2016); however, the reverse trend was observed for the N1P1 amplitude, where along with the duration of the observation period, the correlation between the height of the N1P1 amplitude and the RNFL thickness was significant—in patients with lower N1P1 amplitude, a thinner RNFL was observed. Interestingly, the extent of the initial RGC damage significantly influenced the course of chronic thickness loss of RNFL (Nurieva et al. 2016). During the 4 year follow-up period, a significant thinning of RNFL, especially in the temporal quadrant, was present in repeated OCT studies in 31% of patients (Nurieva et al. 2018). The factor that most strongly correlated with decreasing RNFL thickness in subsequent years was lower arterial blood pH at admission; however, for a higher serum creatinine concentration and a lower serum ethanol concentration, a significant relationship was also revealed (Nurieva et al. 2018). Interestingly, it was shown that patients with ApoE polymorphism showed a more pronounced tendency to develop RNFL thinning and prolongation of initial P1 latency compared to patients without this polymorphism (Nurieva et al. 2018). In addition, a clear preference to impaired visual function, as well as abnormalities in MRI of the brain, the presence of necrosis and hemorrhages, was found in this group (Nurieva et al. 2018).
General prognosis
Many factors that adversely affect the survival prognosis in methanol-intoxicated patients have been identified, including high serum lactate and potassium levels, lower peripheral blood pH, lower serum bicarbonate levels, and higher osmolar and anion gaps at presentation (Noor et al. 2020). Additionally, higher mortality was also found in patients with more prolonged exposure to methanol, as well as in the older age (Ma et al. 2019). Moreover, coma upon admission to the hospital and lack of simultaneous ethanol consumption were also negative factors (Zakharov et al. 2014). Therefore, due to the multiplicity and diversity of prognostic factors, mortality in the acute phase of methanol intoxication ranges from 18 to 44% (Noor et al. 2020).
Studies investigating 6 year mortality after methanol poisoning in patients suffering from Me-ION in both the Czech (Zakharov et al. 2020) and Estonian (Paasma et al. 2009) populations showed 18% and 30% death rate values, respectively. What is worth emphasizing, in the study by Zakharov et al., 47% of deaths were associated with the occurrence of neoplastic, which was probably related to the carcinogenic effect of acute exposure to methanol and its metabolites, especially formaldehyde, and secondary, significantly increased oxidative stress (Zakharov et al. 2020). In the Estonian population, characterized by a similar age structure, but with a higher ratio of females to males compared to the Czech population (31/69 vs. 20/80%), it was shown that the dominant cause of death in the studied group was alcohol intoxication, which occurred in 27% of patients (Paasma et al. 2009). Thus, the results of the above observations show that survivors should be subject to comprehensive supervision and multidisciplinary medical care in further years after Me-ION.
Conclusions
Although, Me-ION has been known for many years and is not a rare problem for clinicians and researchers worldwide. The mechanisms determining the occurrence of pathological changes in its course have not been fully understood, which is the reason for the lack of available effective treatment methods that would protect patients against dramatic visual consequences of methanol poisoning. The mechanism of damage to the structures of the visual system, in particular, the optic nerve and the retina, is multifactorial; therefore it is difficult to develop targeted therapeutic methods of treatment. Patients affected by Me-ION require comprehensive medical care also in the years following poisoning due to adverse health consequences leading to a significant deterioration in the quality of life. Therefore, intensive efforts to reduce the rate of methanol poisoning based on both social education and the development of new therapies and strategies for managing Me-ION patients should be continued.
References
Abrishami M, Khalifeh M, Shoayb M, Abrishami M (2011) Therapeutic effects of high-dose intravenous prednisolone in methanol-induced toxic optic neuropathy. J Ocul Pharmacol Ther 27:261–263
Ahiskali I, Pinar CL, Kiki M, Cankaya M, Kunak CS, Altuner D (2019) Effect of taxifolin on methanol-induced oxidative and inflammatory optic nerve damage in rats. Cutan Ocul Toxicol 38:384–389
Barceloux DG, Randall Bond G, Krenzelok EP, Cooper AVJ (2002) American academy of clinical toxicology practice guidelines on the treatment of methanol poisoning. J Toxicol ClinToxicol 40:415–446
Barták M, Rogalewicz V, Doubek J, Šejvl J, Petruželka B, Zakharov S, Miovský M (2021) Estimation of long-term costs of postacute care in survivors of the methanol poisoning outbreak. BMJ Open. https://doi.org/10.1136/bmjopen-2020-043037
Baumbach GL, Cancilla PA, Martin-Amat G, Tephly TR, McMartin KE, Makar AB, Hayreh MS, Hayreh SS (1977) Methyl alcohol poisoning. IV. Alterations of the morphological findings of the retina and optic nerve. Arch Ophthalmol 95:1859–1865
Bennett IL Jr, Cary FH, Mitchell GL Jr, Cooper MN (1953) Acute methyl alcohol poisoning: a review based on experiences in an outbreak of 323 cases. Medicine (baltimore) 32:431–463
Benton CD Jr, Calhoun EP Jr (1953) The ocular effects of methyl alcohol poisoning; report of a catastrophe involving 320 persons. Am J Ophthalmol 36:1677–1685
Blomstrand R, Ingemansson SO (1984) Studies on the effect of 4-methylpyrazole on methanol poisoning using the monkey as an animal model: with particular reference to the ocular toxicity. Drug Alcohol Depend 13:343–355
Brahmi N, Blel Y, Abidi N, Kouraichi N, Thabet H, Hedhili A, Amamou M (2007) Methanol poisoning in Tunisia: report of 16 cases. Clin Toxicol (phila) 45:717–720
Chen JM, Zhu GY, Xia WT, Zhao ZQ (2012) Proteomic analysis of rat retina after methanol intoxication. Toxicology 293:89–96
Chen JM, Zhu GY, Zhao ZQ, Xia WT (2013) Electroretinogram and histopathologic changes of the retina after methanol intoxication. Fa Yi Xue Za Zhi 29:5–11 (Abstract)
Choi JH, Lee SK, Gil YE, Ryu J, Jung-Choi K, Kim H, Choi JY, Park SA, Lee HW, Yun JY (2017) Neurological complications resulting from non-oral occupational methanol poisoning. J Korean Med Sci 32:371–376
Chung JY, Ho CH, Chen YC, Chen JH, Lin HJ, Wang JJ, Hsu CC, Huang CC (2018) Association between acute methanol poisoning and subsequent mortality: a nationwide study in Taiwan. BMC Public Health 18:985
Cursiefen C, Bergua A (2002) Acute bilateral blindness caused by accidental methanol intoxication during fire “eating.” Br J Ophthalmol 86:1064–1065
Daniele LL, Caughey J, Volland S, Sharp RC, Dhingra A, Williams DS, Philip NJ, Boesze-Battaglia K (2019) Peroxisome turnover and diurnal modulation of antioxidant activity in retinal pigment epithelia utilizes microtubule-associated protein 1 light chain 3B (LC3B). Am J Physiol Cell Physiol 317:1194–1204
Desai T, Sudhalkar A, Vyas U, Khamar B (2013) Methanol poisoning: predictors of visual outcomes. JAMA Ophthalmol 131:358–364
Desmet KD, Paz DA, Corry JJ, Eells JT, Wong-Riley MT, Henry MM, Buchmann EV, Connelly MP, Dovi JV, Liang HL, Henshel DS, Yeager RL, Millsap DS, Lim J, Gould LJ, Das R, Jett M, Hodgson BD, Margolis D, Whelan HT (2006) Clinical and experimental applications of NIR-LED photobiomodulation. Photomed Laser Surg 24:121–128
Dethlefs R, Naraqi S (1978) Ocular manifestations and complications of acute methyl alcohol intoxication. Med J Aust 2:483–485
Doreen B, Eyu P, Okethwangu D, Biribawa C, Kizito S, Nakanwagi M, Nguna J, Nkonwa IH, Opio DN, Aceng FL, Alitubeera PH, Kadobera D, Kwesiga B, Bulage L, Ario AR, Zhu BP (2020) Fatal Methanol poisoning caused by drinking adulterated locally distilled alcohol: Wakiso District, Uganda, 2017. J Environ Public Health. https://doi.org/10.1155/2020/5816162
Dorokhov YL, Shindyapina AV, Sheshukova EV, Komarova TV (2015) Metabolic methanol: molecular pathways and physiological roles. Physiol Rev 95:603–644
Eells JT, Makar AB, Noker PE, Tephly TR (1981) Methanol poisoning and formate oxidation in nitrous oxide-treated rats. J Pharmacol Exp Ther 217:57–61
Eells JT, Salzman MM, Lewandowski MF, Murray TG (1996) Formate-induced alterations in retinal function in methanol-intoxicated rats. Toxicol Appl Pharmacol 140:58–69
Eells JT, Henry MM, Lewandowski MF, Seme MT, Murray TG (2000) Development and characterization of a rodent model of methanol-induced retinal and optic nerve toxicity. Neurotoxicology 21:321–330
Eells JT, Henry MM, Summerfelt P, Wong-Riley MT, Buchmann EV, Kane M, Whelan NT, Whelan HT (2003) Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci USA 100:3439–3444
El-Din A, Gawad E, Amal EI (2011) Effect of methanol intoxication on the function of retina of rabbit. J Am Sci 7:491–496
Fu J, Jiao J, Weng K, Yu D, Li R (2017) Zebrafish methanol exposure causes patterning defects and suppressive cell proliferation in retina. Am J Transl Res 9:2975–2983
Fujihara M, Kikuchi M, Kurimoto Y (2006) Methanol-induced retinal toxicity patient examined by optical coherence tomography. Jpn J Ophthalmol 50:239–241
Gabay O, Talmon A, Tsumi E, Bartal C (2018) Reversal of severe methanol induced visual impairment due to prompt hemodialysis. J Clin Toxicol 8:379
Galvez-Ruiz A, Elkhamary SM, Asghar N, Bosley TM (2015) Visual and neurologic sequelae of methanol poisoning in Saudi Arabia. Saudi Med J 36:568–574
Garner CD, Lee EW, Terzo TS, Louis-Ferdinand RT (1995) Role of retinal metabolism in methanol-induced retinal toxicity. J Toxicol Environ Health 44:43–56
Gauvin M, Dorfman AL, Trang N, Gauthier M, Little JM, Lina JM, Lachapelle P (2016) Assessing the contribution of the oscillatory potentials to the genesis of the photopic ERG with the discrete wavelet transform. Bio Med Res Int. https://doi.org/10.1155/2016/2790194
Glorieux C, Calderon P (2017) Catalase, a remarkable enzyme: targeting the oldest anti-oxidant enzyme to find a new cancer treatment approach. Biol Chem 398:1095–1108
González-Quevedo A, Obregón F, Urbina M, Roussó T, Lima L (2002) Effect of chronic methanol administration on amino acids and monoamines in retina, optic nerve, and brain of the rat. Toxicol Appl Pharmacol 185:77–84
González-Quevedo A, Santiesteban-Freixas R, Eells JT, Lima L, Sadun AA (2018) Cuban epidemic neuropathy: insights into the toxic-nutritional hypothesis through international collaboration. MEDICC Rev 20:27–31
Grzybowski A, Zülsdorff M, Wilhelm H, Tonagel F (2015) Toxic optic neuropathies: an updated review. Acta Ophthalmol 93:402–410
Gulen M, Satar S, Avci A, Acehan S, Orhan U, Nazik H (2020) Methanol poisoning in Turkey: two outbreaks, a single center experience. Alcohol 88:83–90
Hamon A, Roger J, Yang X, Perron M (2016) Müller glial cell-dependent regeneration of the neural retina: an overview across vertebrate model systems. Dev Dyn 245:727–738
Hassanian-Moghaddam H, Nikfarjam A, Mirafzal A, Saberinia A, Nasehi AA, Masoumi Asl H, Memaryan N (2015) Methanol mass poisoning in Iran: role of case finding in outbreak management. J Public Health (oxf) 37:354–359
Hayasaka Y, Hayasaka S, Hiraki S, Kadoi C, Nagaki Y, Matsumoto M (2000) Serum methanol levels in subjects with or without optic nerve head disease. Ophthalmic Res 32:299–304
Hayasaka Y, Hayasaka S, Nagaki Y (2001) Ocular changes after intravitreal injection of methanol, formaldehyde, or formate in rabbits. Pharmacol Toxicol 89:74–78
Hayreh SS (1989) Optic nerve involvement in methanol poisoning. Br J Ophthalmol 73:238–240
Hedges TR 3rd, Hirano M, Tucker K, Caballero B (1997) Epidemic optic and peripheral neuropathy in Cuba: a unique geopolitical public health problem. Surv Ophthalmol 41:341–453
Hovda KE, Hunderi OH, Rudberg N, Froyshov S, Jacobsen D (2004) Anion and osmolal gaps in the diagnosis of methanol poisoning: clinical study in 28 patients. Intensive Care Med 30(9):1842–1846
Hovda KE, Hunderi OH, Tafjord AB, Dunlop O, Rudberg N, Jacobsen D (2005) Methanol outbreak in Norway 2002–2004: epidemiology, clinical features and prognostic signs. J Intern Med 258:181–190
Huang L, Huang QY, Chen HB, Huang FS, Huang HQ (2011) Differential proteins of the optic ganglion in octopus vulgaris under methanol stress revealed using proteomics. Appl Biochem Biotechnol 165:978–988
Icel E, Suleyman H, Yazici GN, Bakan N, Sunar M (2020) Effects of adenosine triphosphate on methanol-induced experimental optic nerve damage in rats: biochemical and histopathological evaluation. Cutan Ocul Toxicol 39:244–248
Ingemansson SO (1984) Clinical observations on ten cases of methanol poisoning with particular reference to ocular manifestations. Acta Ophthalmol (copenh) 62:15–24
İşcan Y, Coşkun Ç, Öner V, Türkçü FM, Taş M, Alakuş MF (2013) Bilateral total optic atrophy due to transdermal methanol intoxication. Middle East Afr J Ophthalmol 20:92
Jayaram H, Jones MF, Eastlake K, Cottrill PB, Becker S, Wiseman J, Khaw PT, Limb GA (2014) Transplantation of photoreceptors derived from human Muller glia restore rod function in the P23H rat. Stem Cells Transl Med 3:323–333
Kaewput W, Thongprayoon C, Petnak T, Chewcharat A, Boonpheng B, Bathini T, Vallabhajosyula S, Cheungpasitporn W (2021) Inpatient Burden and mortality of methanol intoxication in the United States. Am J Med Sci 361:69–74
Kavet R, Nauss KM (1990) The toxicity of inhaled methanol vapors. Crit Rev Toxicol 21:21–50
Khalili MR, Sadati MS, Jahanbani-Ardakani H (2021) Outbreak of methanol-induced optic neuropathy amid COVID-19 pandemic. Graefes Arch Clin Exp Ophthalmol 259:1375–1376
Klein KA, Warren AK, Baumal CR, Hedges TR (2017) Optical coherence tomography findings in methanol toxicity. Int J Retin Vitr 3:1–6
Koehrer P, Creuzot-Garcher C, Bron AM (2011) Methanol poisoning: two case studies of blindness in Indonesia. Int Ophthalmol 31:517–524
Kowalski T, Verma J, Greene SL, Curtin J (2019) Methanol toxicity: a case of blindness treated with adjunctive steroids. Med J Aust 210:14–15
Kraut JA (2016) Approach to the treatment of methanol intoxication. Am J Kidney Dis 68:161–167
Kumar M, Kaeley N, Nagasubramanyam V, Bhardwaj BB, Kumar S, Kabi A, Arora P, Dhar M (2019) Single center experience of managing methanol poisoning in the hilly state of uttarakhand: a cross sectional study. Int J Crit Ill Inj Sci 9:172
Lee EW, Garner CD, Terzo TS (1994) A rat model manifesting methanol-induced visual dysfunction suitable for both acute and long-term exposure studies. Toxicol Appl Pharmacol 128:199–206
Lenkowski JR, Raymond PA (2014) Müller glia: stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retin Eye Res 40:94–123
Lincoff NS, Odel JG, Hirano M (1993) “Outbreak” of optic and peripheral neuropathy in cuba? JAMA 270:511–518
Liu DM, Zhou S, Chen JM, Peng SY, Xia WT (2016) The intoxication effects of methanol and formic acid on rat retina function. J Ophthalmol. https://doi.org/10.1155/2016/4087096
Ma Z, Jiang H, Wang J (2019) Clinical analysis of severe visual loss caused by inhalational methanol poisoning in a chronic process with acute onset: a retrospective clinical analysis. BMC Ophthalmol 19:1–7
MacFarlan JF (1855) On methylated spirits and some of its preparations. Pharm J Trans 15:310–315 (Cited according to: Lee et al. 1994)
Martens J, Westhovens R, Verberckmoes R, Delooz H, Daenens P (1982) Recovery without sequelae from severe methanol intoxication. Postgrad Med J 58(681):454–456
Masoud RA, Khalifa EA, Kabeel MA (2016) The role of pulse steroid therapy in cases of methanol induced ocular toxicity. Egypt J Forensic Sci Appli Toxicol 16:37–54
Masson J (2019) Serotonin in retina. Biochimie 161:51–55
McCormick MJ, Mogabgab E, Adams SL (1990) Methanol poisoning as a result of inhalational solvent abuse. Ann Emerg Med 19:639–642
McKellar MJ, Hidajat RR, Elder MJ (1997) Acute ocular methanol toxicity: clinical and electrophysiological features. Aust N Z J Ophthalmol 25:225–230
Moral AR, Çankayalı İ, Sergin D, Boyacılar Ö (2015) Neuromuscular functions on experimental acute methanol intoxication. Turk J Anaesth Reanim 43:337–343
Moschos MM, Gouliopoulos NS, Rouvas A, Ladas I (2013) Vision loss after accidental methanol intoxication: a case report. BMC Res Notes 6:479
Murray TG, Burton TC, Rajani C, Lewandowski MF, Burke JM, Eells JT (1991) Methanol poisoning. A rodent model with structural and functional evidence for retinal involvement. Arch Ophthalmol 109:1012–1016
Naeser P (1988) Optic nerve involvement in a case of methanol poisoning. Br J Ophthalmol 72:778–781
Noor JM, Hawari R, Mokhtar MF, Yussof SJ, Chew N, Norzan NA, Rahimi R, Ismail Z, Singh S, Baladas J, Hashim NH, Mohadam NIK (2020) Pathmanathan MD (2020) Methanol outbreak: a Malaysian tertiary hospital experience. INTJEM 13:6
Nurieva O, Kotikova K, Urban P, Pelclova D, Petrik V, Navratil T, Zakharov S (2016) Prevalence, dynamics, and biochemical predictors of optic nerve remyelination after methanol-induced acute optic neuropathy: a 2-year prospective study in 54 patients. Monatsh Chem 147:239–249
Nurieva O, Diblik P, Kuthan P, Sklenka P, Meliska M, Bydzovsky J, Heissigerova J, Urban P, Kotikova K, Navratil T, Komarc M, Seidl Z, Vaneckova M, Pelclova D, Zakharov S (2018) Progressive chronic retinal axonal loss following acute methanol-induced optic neuropathy: four-year prospective cohort study. Am J Ophthalmol 191:100–115
Paasma R, Hovda KE, Tikkerberi A, Jacobsen D (2007) Methanol mass poisoning in Estonia: outbreak in 154 patients. Clin Toxicol (phila) 45:152–157
Paasma R, Hovda KE, Jacobsen D (2009) Methanol poisoning and long term sequelae—a six years follow-up after a large methanol outbreak. BMC Clin Pharmacol 9:5
Pakdel F, Sanjari MS, Naderi A, Pirmarzdashti N, Haghighi A, Kashkouli MB (2018) Erythropoietin in treatment of methanol optic neuropathy. J Neuroophthalmol 38:167–171
Pakdel F (2019) Erythropoietin in Methanol Associated Optic Neuropathy: A Phase-2 Clinical Trial. InvestigOphthalmol Vis Sci. 2019 ARVO Annual Meeting, Vancouver, Canada, April 28 - May 2, 2019 [Conference abstract]
Pakravan M, Esfandiari H, Sanjari N, Ghahari E (2016) Erythropoietin as an adjunctive treatment for methanol-induced toxic optic neuropathy. Am J Drug Alcohol Abuse 42:633–639
Parthasarathy NJ, Kumar RS, Manikandan S, Narayanan GS, Kumar RV, Devi RS (2006) Effect of methanol-induced oxidative stress on the neuroimmune system of experimental rats. Chem Biol Interact 161:14–25
Parthasarathy NJ, Srikumar R, Manikandan S, Narayanan GS, Devi RS (2007) Effect of methanol intoxication on specific immune functions of albino rats. Cell Biol Toxicol 23:177–187
Permaisuari N, Rahmawati NA, Nusanti S, Thamrin H (2019) Efficacy of high-dose intravenous steroid treatment in methanol-induced optic neuropathy: a systematic review. Int J App Pharm 11(Special Issue 6):97–100
Plaziac C, Lachapelle P, Casanova C (2003) Effects of methanol on the retinal function of juvenile rats. Neurotoxicology 24:255–260
Potts AM, Praglin J, Farkas I, Lowell MS, Orbison L, Chickering D (1955) Studies on the visual toxicity of methanol. VIII. Additional observations on methanol poisoning in the primate test object. Am J Ophthalmol 40:76–82
Ranjan R, Kushwaha R, Gupta RC, Khan P (2014) An unusual case of bilateral multifocal retinal pigment epithelial detachment with methanol-induced optic neuritis. J Med Toxicol 10:57–60
Rasheed R, Youakim M, Yehia M (2017) Possible protective effect of ethanol on acute methanol intoxicated retina in rats: a histological and ultrastructural study. J Med Histol 1:146–153
Rostrup M, Edwards JK, Abukalish M, Ezzabi M, Some D, Ritter H, Menge T, Abdelrahman A, Rootwelt R, Janssens B, Lind K, Paasma R, Hovda KE (2016) The methanol poisoning outbreaks in Libya 2013 and Kenya 2014. PLoS ONE. https://doi.org/10.1371/journal.pone.0152676
Rotenstreich Y, Assia EI, Kesler A (1997) Late treatment of methanol blindness. Br J Ophthalmol 81:416–417
Ruedemann AD Jr (1962) The electroretinogram in chronic methyl alcohol poisoning in human beings. Am J Ophthalmol 54:34–53
Rulisek J, Waldauf P, Belohlavek J, Balik M, Kotikova K, Hlusicka J, Vaneckova M, Seidl Z, Diblik P, Bydzovsky J, Heissigerova J, Urban P, Miovsky M, Sejvl J, Pelclova D, Zakharov S (2020) Health-related quality of life determinants in survivors of a mass methanol poisoning outbreak: six-year prospective cohort study. Clin Toxicol (phila) 58:870–880
Sadun A (1998) Acquired mitochondrial impairment as a cause of optic nerve disease. Trans Am Ophthalmol Soc 96:881–923
Sadun AA, Martone JF, Muci-Mendoza R, Reyes L, DuBois L, Silva JC, Roman G, Caballero B (1994) Epidemic optic neuropathy in Cuba. Eye Findings Arch Ophthalmol 112:691–699
Saoudi M, Jebahi S, Jamoussi K, Ben Salah G, Kallel C, El Feki A (2011) Haematological and biochemical toxicity induced by methanol in rats: ameliorative effects of Opuntia vulgaris fruit extract. Hum Exp Toxicol 30:1963–1971
Sefidbakht S, Lotfi M, Jalli R, Moghadami M, Sabetian G, Iranpour P (2020) Methanol toxicity outbreak: when fear of COVID-19 goes viral. Emerg Med J 37:416
Seme MT, Summerfelt P, Henry MM, Neitz J, Eells JT (1999) Formate-induced inhibition of photoreceptor function in methanol intoxication. J Pharmacol Exp Ther 289:361–370
Seme MT, Summerfelt P, Neitz J, Eells JT, Henry MM (2001) Differential recovery of retinal function after mitochondrial inhibition by methanol intoxication. Investig Ophthalmol vis Sci 42:834–841
Setiohadji B, Irfani I, Rifada M, Virgana R, Kartasasmita AS (2018) The superoxide dismutase mimetic TEMPOL and Its effect on retinal ganglion cells in experimental methanol-intoxicated rats. Ophthalmol Ther 7:167–172
Sharma M, Volpe NJ, Dreyer EB (1999) Methanol-induced optic nerve cupping. Arch Ophthalmol 117:286
Sharma R, Marasini S, Sharma AK, Shrestha JK, Nepal BP (2012) Methanol poisoning: ocular and neurological manifestations. Optom vis Sci 89:178–182
Sharpe JA, Hostovsky M, Bilbao JM, Rewcastle NB (1982) Methanol optic neuropathy: a histopathological study. Neurology 32:1093–1100
Shen Y, Liu XL, Yang XL (2006) N-methyl-D-aspartate receptors in the retina. Mol Neurobiol 34:163–179
Shin YW, Uhm KB (2011) A case of optic nerve atrophy with severe disc cupping after methanol poisoning. Korean J Ophthalmol 25:146–150
Shukla M, Shikoh I, Saleem A (2006) Intravenous methylprednisolone could salvage vision in methyl alcohol poisoning. Indian J Ophthalmol. https://doi.org/10.4103/0301-4738.21628
Singhal S, Bhatia B, Jayaram H, Becker S, Jones MF, Cottrill PB, Khaw PT, Salt TE, Limb GA (2012) Human Müller glia with stem cell characteristics differentiate into retinal ganglion cell (RGC) precursors in vitro and partially restore RGC function in vivo following transplantation. Stem Cells Transl Med 1:188–199
Sodhi PK, Goyal JL, Mehta DK (2001) Methanol-induced optic neuropathy: treatment with intravenous high dose steroids. Int J Clin Pract 55:599–602
Stunkel L, Van Stavern G (2018) Steroid treatment of optic neuropathies. Asia Pac J Ophthalmol 7:218–228
Su L-J, Zhang J-H, Gomez H, Murugan R, Hong X, Xu D, Jiang F, Peng Z-Y (2019) Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid Med Cell Longev. https://doi.org/10.1155/2019/5080843
Sullivan-Mee M, Solis K (1998) Methanol-induced vision loss. J Am Optom Assoc 69:57–65
Tanrivermis Sayit A, Aslan K, Elmali M, Gungor I (2016) Methanol-induced toxic optic neuropathy with diffusion weighted MRI findings. Cutan Ocul Toxicol 35:337–340
Taşlı NG, Çimen FK, Karakurt Y, Uçak T, Mammadov R, Süleyman B, Kurt N, Süleyman H (2018) Protective effects of Rutin against methanol induced acute toxic optic neuropathy: an experimental study. Int J Ophthalmol 11:780–785
Treichel JL, Henry MM, Skumatz CM, Eells JT, Burke JM (2003) Formate, the toxic metabolite of methanol, in cultured ocular cells. Neurotoxicology 24:825–834
Vera MS, Simón MV, Prado Spalm FH, Ayala-Peña VB, German OL, Politi LE, Santiago Valtierra FX, Rotstein NP (2021) Ceramide-1-phosphate promotes the migration of retina Müller glial cells. Exp Eye Res. https://doi.org/10.1016/j.exer.2020.108359
Yang CS, Tsai WJ, Lirng JF (2005) Ocular manifestations and MRI findings in a case of methanol poisoning. Eye 19:806–809
Yieh FS, Chou PI (2002) Bilateral methanol optic neuropathy after intravenous administration. Jtoxicol Cutaneous OculToxicol 21:169–174
Yip L, Bixler D, Brooks DE, Clarke KR, Datta SD, Dudley S Jr, Komatsu KK, Lind JN, Mayette A, Melgar M, Pindyck T, Schmit KM, Seifert SA, Shirazi FM, Smolinske SC, Warrick BJ, Chang A (2020) Serious adverse health events, including death, associated with ingesting alcohol-based hand sanitizers containing methanol—Arizona and New Mexico, May–June 2020. MMWR Morb Mortal Wkly Rep 69:1070–1073
Yoo RM, Shin HJ, Shin KC (2016) A case of complete visual recovery after methanol intoxication. Ann Optom Contact Lens 15:161–165
Yu-Wai-Man P, Votruba M, Moore AT, Chinnery PF (2014) Treatment strategies for inherited optic neuropathies: past, present and future. Eye (lond) 28:521–537
Zakharov S, Pelclova D, Urban P, Navratil T, Diblik P, Kuthan P, Hubacek JA, Miovsky M, Klempir J, Vaneckova M, Seidl Z, Pilin A, Fenclova Z, Petrik V, Kotikova K, Nurieva O, Ridzon P, Rulisek J, Komarc M, Hovda KE (2014) Czech mass methanol outbreak 2012: epidemiology, challenges and clinical features. Clin Toxicol (phila) 52:1013–1024
Zakharov S, Pelclova D, Diblik P, Urban P, Kuthan P, Nurieva O, Kotikova K, Navratil T, Komarc M, Belacek J, Seidl Z, Vaneckova M, Hubacek JA, Bezdicek O, Klempir J, Yurchenko M, Ruzicka E, Miovsky M, Janikova B, Hovda KE (2015) Long-term visual damage after acute methanol poisonings: longitudinal cross-sectional study in 50 patients. Clin Toxicol (phila) 53:884–892
Zakharov S, Rulisek J, Hlusicka J, Kotikova K, Navratil T, Komarc M, Vaneckova M, Seidl Z, Diblik P, Bydzovsky J, Heissigerova J, Zogala D, Hubacek JA, Miovsky M, Sejvl J, Vojtova L, Pelclova D (2020) The impact of co-morbidities on a 6-year survival after methanol mass poisoning outbreak: possible role of metabolic formaldehyde. Clin Toxicol (phila) 58:241–253
Zamani N, Hassanian-Moghaddam H, Shojaei M, Rahimian S (2018) Evaluation of the effect of erythropoietin + corticosteroid versus corticosteroid alone in methanol-induced optic nerve neuropathy. Cutan Ocul Toxicol 37:186–190
Zarenezhad A, Esf A, Aliabadi A (2013) Preventive effects of silymarin in retinal intoxication with methanol in rat: transmission electron microscope study. Afr J Pharm Pharmacol 7:1757–1761
Zhai R, Zheng N, Rizak J, Hu X (2016) Evidence for conversion of methanol to formaldehyde in nonhuman primate brain. Anal Cell Pathol. https://doi.org/10.1155/2016/4598454
Zhao XJ, Lu L, Li M, Yang H (2015) Ophthalmic findings in two cases of methanol optic neuropathy with relapsed vision disturbance. Int J Ophthalmol 8:427
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liberski, S., Kaluzny, B.J. & Kocięcki, J. Methanol-induced optic neuropathy: a still-present problem. Arch Toxicol 96, 431–451 (2022). https://doi.org/10.1007/s00204-021-03202-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-021-03202-0