Abstract
Cadmium (Cd) pollution in agricultural soils induces oxidative stress in plants that in turn is the foremost limiting factor for agricultural productivity. In past few decades, plant–metal–microbe interaction is of great interest as an emerging environmentally friendly technology that can be exploited to alleviate metal stress in plants. Considering these, in the present study an endophytic bacterium strain EPS has been isolated from the roots of common bean. The present strain was identified as Stenotrophomonas maltophilia based on 16S rRNA gene sequence. The strain showed Cd tolerance and Cd-adsorption potentials. The inoculation of strain EPS in safflower seeds significantly enhanced the antioxidant defense of plants under Cd-stress conditions through increasing the levels of antioxidant molecules like phenolics, flavonoids and carotenoids as well as improving the activities of the antioxidative enzymes including guaiacol peroxidase (POX), ascorbate peroxidase (APX) and superoxide dismutase (SOD). The output of this study is that strain EPS inoculation mitigates Cd-induced oxidative stress and consequently it may be beneficial, especially in Cd-contaminated crop fields.
Similar content being viewed by others
Introduction
Over wide world, heavy metals accumulation in agricultural soils is a serious problem threats crop production (He et al. 2015; Rizwan et al. 2016). Plants uptake heavy metals from contaminated soils and subsequently transmit along the food chain causing potential threat to animal and human health. (Fryzova et al. 2017). Hyperlevels of heavy metals alter normal plant functions and metabolism causing repression of vital processes such as photosynthesis, respiration, and enzymatic activities (Hossain et al. 2015). On the other hand, high levels of heavy metals can induce excess generation of reactive oxygen species (ROS) as well as cytotoxic compounds, leading to oxidative stress via demolishing the equilibrium between prooxidants and antioxidants within the plant cells (Zengin and Munzuroglu 2005; Hossain et al. 2015; Sytar et al. 2013). This results in cellular damage as well as decreasing plant productivity (Raja et al. 2017).
Cadmium (Cd) is unnecessary element for living organisms, and it is highly toxic to plants and animals even at very low concentrations (Dai et al. 2012). Cadmium mainly originates from industrial processes and phosphate fertilizers, releases into agricultural lands and has long biological half-life (Gill et al. 2013). In plants, the exposure to cadmium induces numerous hazards physiological and growth changes as well as oxidative stress by generating ROS, that react with lipids, proteins, pigments and nucleic acids in the plant cell, leading to cellular damage and consequently decreasing productivity (Romero-Puertas et al. 2004). Furthermore, cadmium also can transfer into human via food chain and can result in kidney, bone and lung diseases (Bernard 2008).
Traditional remediation techniques for heavy metal-contaminated soils are expensive and destructive to environment (Meagher 2000). Therefore, scientists and engineers intensify their efforts to find cost effective and safe technologies (Boyajian and Carreira 1997; Wasay et al. 1998). Most of plant associated microorganisms are metal resistant, whose application in heavy metal-contaminated soils can improve metal immobilization in soils and plant biomass (Ma et al. 2011, 2016). Despite that the applications of some potential bacterial strains to remediate soils contaminated with heavy metals have been reported, it is urgent to search a new microbial resources that can be used efficiently in heavy metals remediation (Tirry et al. 2018).
Safflower (Carthamus tinctorius L.) is herbaceous annual plant belongs to family Asteraceae. It is cultivated from prehistoric times throughout many areas with temperate climates over the world including southern Asia, China, India, Iran and Egypt (Dordas and Sioulas 2008; Weiss 2000). Safflower is commercially used for vegetable oil extraction, as well as in the traditional medicine for the treatment of rheumatism, paralysis, vitiligo, psoriasis and mouth ulcers (Delshad et al. 2018). Moreover, it has numerous pharmacological activities i.e., antioxidant, analgesic, anti-inflammatory and antidiabetic activities (Asgarpanah and Kazemivash 2013).
It has been reported that safflower plants can accumulate high levels of Cd in their roots and leaves (Shi et al. 2010; Namdjoyan et al. 2011). Although, some scientific data exists on the antioxidant defense mechanisms in response to cadmium stress in safflower cultivars (Namdjoyan et al. 2011), to our knowledge, there is no study dealing with alleviation of Cd- induced oxidative stress in safflower using bacteria. Therefore, the present work was designed to investigate the potentiality of the endophytic bacterium Stenotrophomonas maltophilia strain EPS to alleviate Cd-induced oxidative stress in safflower plants.
Materials and methods
Isolation and identification of endophytic bacteria
Healthy fresh roots of common bean plants (Vigna unguiculata L.) were collected in sterile plastic bags from Aswan University greenhouse. Immediately, samples were surface- sterilized using 70% ethanol (30 s) followed by 5% sodium hypochlorite (3 min) and then washed three times with sterilized distilled water (Vincent 1970). Under aseptic conditions, roots were crushed in sterilized saline solution. Loopful of the obtained suspension was streaked on the surface of tryptic soy agar and nutrient agar plates. Plates were incubated at 37 °C for 72 h for the appearance of colonies.
The ribosomal (16S rRNA) gene of the selected strain was amplified using 27F and 1492R primers (Frank et al. 2008) in Applied Biotechnology lab at Ismailia, Egypt. PCR product was sent to SolGent Co., Ltd., South Korea for sequencing. Then, the similarity of the obtained sequence was evaluated based on BLAST outputs using NCBI reference sequence database. Neighbor-joining phylogenetic tree of the strain was constructed using MEGA X 10.1.7 software (Kumar et al. 2018).
Cd tolerance by the strain
The maximum tolerable concentration of cadmium by the strain was determined according to the method of Vashishth and Khanna (2015), with slight modification. Briefly, 10 mL of nutrient broth in glass tubes was supplemented with different concentrations of CdCl2 i.e., 0 (control), 50, 100, 150, 200, 250 and 300 mg L−1. 10 mL of nutrient broth without CdCl2 was used as control. Tubes were inoculated with 1 mL of inoculum (107 CFU mL−1), and incubated for 48 h at 37 °C and 150 rpm. The optical density (OD) was measured at 600 nm. The highest concentration of cadmium (CdCl2) that allowed visible bacterial growth after 48 h of incubation was considered as the maximum tolerable concentration.
Production of exopolysaccharides (EPS) by the strain
In 250 mL conical flasks, 50 mL of nutrient broth was inoculated with 1 mL of bacterial suspension (107 CFU mL−1), and incubated at 37 °C in a rotary shaker at 150 rpm for 48 h. Cultures were centrifuged at 5000 rpm for 15 min. The total content of EPS in the supernatants were estimated using phenol–sulphuric acid method (Dubois et al. 1956).
Cd- adsorption ability of the strain
The ability of the whole culture of the present strain (cells and supernatant) for adsorping cadmium was evaluated using the method of Du et al. (2016). 100 mL of the whole culture broth contained 50 and 100 mg L−1 of CdCl2 was shaken at 120 rpm and 37 °C for 24 h. Cells were then removed by centrifugation. Concentration of the residual, non-adsorbed metal ion in the solution was estimated by atomic absorption spectrophotometer (Thermo Scientific™ iCE™ 3000). Experiment was performed in triplicate. The adsorption efficiency (%) was calculated according to the following formula:
where Cdi and Cde are the concentration of initial and equilibrium Cd ion in the solution (mg L−1) respectively.
Seed inoculation and pot experiment
Seeds of safflower (cv. Giza-1) were obtained from Faculty of Agriculture and Natural Resources, Aswan University. Seeds were surface sterilized with 70% ethanol for 3 min, rinsed three times with sterilized distilled water. Seeds thereafter were soaked in a freshly prepared bacterial suspension (108 CFU mL−1) for 1 h, and left to dry before sowing. Seeds used for control were soaked in sterilized distilled water.
Seeds were sown in pots containing an autoclaved mixture of clay and sand (1:1 w/w), with maintaining field capacity at 90%. Pots were kept under normal climatic conditions. After 3 weeks of sowing, five homogenous plants in each pot were subjected to three Cd treatments including 0 (control), 50 and 100 mg L−1 of CdCl2. After 3 weeks of cadmium exposure, healthy expanded leaf samples were collected, frozen and then used for measuring the defensive non-enzymatic and enzymatic antioxidant activities. The experiment was repeated twice.
Assessments of non-enzymatic antioxidants
Total phenolics
The Folin-Ciocalteu assay described by Singleton et al. (1999) was followed to determine the total phenolic compounds in the leaves extracts. Absorbance was read at 700 nm, and the content of total phenolics was expressed as mg gallic acid equivalents per gram of fresh weight using gallic acid as a reference.
Total flavonoids
Aluminum chloride method according to Chang et al. (2002) was used for quantifying the total contents of flavonoids of the extracts. The absorbance was recorded at wavelength 510 nm. The concentration of flavonoids was calculated from quercetin calibration curve as mg quercetin equivalents per gram of fresh weight.
Total carotenoids
Pigments were extracted from fresh leaves and their contents were estimated as described by Lichtenthaler and Wellburn (1983). One gram of fresh leaves was macerated in 80% acetone, the supernatant was filtered and makeup to 50 mL with the solvent. The total contents of chlorophylls a (Chl a), chlorophylls b (Chl b) and carotenoids were measured by reading the absorbance at wavelengths 646, 663 and 440.5 nm respectively. The content of each pigment was calculated in mg per gram of fresh weight using the following equations:
Total antioxidant capacity
Total antioxidant capacity of the ethanolic extracts of the leaves was measured per gram of fresh weight as mg ascorbic acid equivalents using ascorbic acid standard curve, according to phosphomolybdnum assay (Prieto et al. 1999).
Assessments of enzymatic antioxidants
Antioxidant enzymes were extracted from fresh leaves according to Cavalcanti et al. (2004) with slight modification. One gram of fresh leaves was homogenized using a mortar in 10 mL of extraction buffer containing 0.2 M of potassium phosphate buffer (pH 7.2), 0.1 mM EDTA and 1 mM phenylmethylsulfonyl fluoride as proteinase inhibitor. The homogenate was filtered. The obtained filtrate was used for enzymatic assays.
Catalase (CAT) activity
Catalase activity was estimated by the method of Kato and Shimizu (1987). To 3 mL of the reaction mixture containing 50 mM potassium phosphate buffer (pH 7.0) and 20 mM H2O2, 100 µl of enzymatic extract was added. The decrease in H2O2 was followed as decline in optical density at 240 nm. Catalase activity was calculated with the extinction coefficient of H2O2 (40 mM−1 cm−1), and expressed as 1 μmol of H2O2 decomposed per minute under assay conditions.
Guaiacol peroxidase (POX) activity
The activity of guaiacol peroxidase enzyme was determined following the method of Kim and Yoo (1996). Briefly, the reaction mixture contained 0.2 mL of enzyme extract, 0.8 mL of phosphate buffer (0.2 M, pH 7.2), 1 mL of guaiacol (15 mM) and 1 mL of hydrogen peroxide (3 mM) was incubated for 10 min at 30 °C. Reaction was terminated using 0.5 mL of H2SO4 (5%), and the absorbance was read at 470 nm. POX activity was calculated using the extinction coefficient of oxidation product (tetraguaiacol), (ε470 = 26.6 mM cm−1) as follow:
Ascorbate peroxidase (APX) activity
Ascorbate peroxidase activity was evaluated according to Senthilkumar et al. (2021). To 0.8 mL of a reaction mixture contained potassium phosphate buffer (50 mM), ascorbic acid (0.5 mM), H2O2 (1.0 mM) and EDTA (0.1 mM), 0.2 mL of the enzyme extract was added. After 30 s the decrease in absorbance at 290 nm was followed up to 60 s with an interval of 15 s. One unit of enzyme activity was expressed as the amount of enzyme required to oxidize 1 μmoL of ascorbic acid per minute with absorbance coefficient 2.8 mM cm at 290 nm.
Superoxide dismutase (SOD) activity
Superoxide dismutase activity was estimated according to Van Rossun et al. (1997). Three mL of reaction mixture contained 50 mM sodium phosphate buffer (pH 7.6), 0.1 mM EDTA, 50 mM sodium carbonate, 50 μM nitroblue tetrazolium (NBT), 10 μM riboflavin, 12 mM l-methionine and 100 μl of crude extract. Tubes contained the same reaction mixture without enzyme extract used as control. The tubes were placed under two 15 W fluorescent lamps for 15 min to start the reaction. The absorbance was recorded at 560 nm. One unit of SOD activity was defined as the amount of enzyme which reduced the absorbance to 50% compared with the control.
Estimation of hydrogen peroxide (H2O2) content in safflower leaves
To evaluate the H2O2 content of the leaves, the method of Velikova et al. (2000) was followed. One gram of fresh leaves was homogenated in 10 mL trichloroacetic acid (0.1%) using a mortar and pestle, and then centrifuged. To 0.5 mL of the supernatant, 0.5 mL of potassium phosphate buffer (pH 7.0) and 1 mL of 1 M KI were added. The mixture was vortexed, and the absorbance was read at 390 nm. A calibration curve of different concentrations (µmol) of 30% (v/v) H2O2 was used as standard.
Statistical analysis
Experimental data were compared by one-way analysis of variance (ANOVA) and Tukey's HSD test using Minitab software (version 18.1). Values were expressed as means ± standard errors (SEs) of three biological replicates obtained from two independent experiments. Different letter alphabets above the graphs indicate significant differences at p ≤ 0.05 between inoculated and non-inoculated plants according to Tukey's HSD test, while similar letters indicate no significant result.
Results
Bacterial identification
The selected strain was coded as EPS. The NCBI- BLAST analysis of strain EPS sequence showed closely similarity with percent identity of 100% to Stenotrophomonas maltophilia strain IAM 12,423 (MN240936) (Fig. 1). The 16S rRNA gene sequence of strain EPS was deposited to NCBI GenBank with accession number (OK584766).
Cd tolerance of the strain
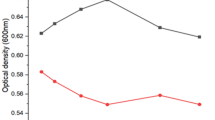
The growth of strain EPS was estimated after 48 h of incubation at different concentrations of CdCl2. It was observed that the maximum tolerable concentration was 200 mg L−1 CdCl2, above this concentration the growth was dramatically declined (Fig. 2).
Production of exopolysaccharides (EPS) by the strain
Strain EPS exhibited its ability to produce significant amount of EPS. Acorrding to phenol–sulphuric acid assay, the EPS production by the strain was 1.103 ± 0.153 mg glucose equivalent mL−1.
Cd- adsorption ability of the strain
Cd-adsorption efficiency was evaluated using the whole culture of the strain (cells and supernatant) supplemented with 50 and 100 mg L−1 CdCl2. It was detected that the Cd- adsorption efficiency by the strain was 95.42% and 89.96% in 50 and 100 mg L−1 Cd-supplemented culture respectively.
Effect of bacterial inoculation on antioxidant defense of safflower under Cd stress
This study showed that the inoculation of safflower seeds with strain EPS significantly enhanced the antioxidant defense of the plants under Cd stress which directly reflected on plant morphology (Fig. 3).
Non-enzymatic antioxidants levels
In the present study, the total phenolics significantly increased (f = 9.11; p = 0.0129) with increasing Cd concentration in inoculated plants comparing to non-inoculated plants (Fig. 4a). On the other hand, the inoculation with strain EPS enhanced the total flavonoids content at all the tested Cd concentrations (Fig. 4b). It was found that the content of total flavonoids was increased in inoculated plants by 38.9 and 49.4% over the non-inoculated plants at Cd concentrations of 50 and 100 mg L−1 respectively.
Effect of bacterial inoculation on a total phenolics, b total flavonoids, c total Carotenoids and d total antioxidant capacity (mg g−1 FW) under Cd stress. Values are means ± SEs of three independent replicates (n = 3). Different letter alphabets above the graphs indicate significant differences at p ≤ 0.05 between inoculated and non-inoculated plants according to Tukey's HSD test, while similar letters indicate no significant result
Although the content of carotenoids of non-inoculated plants under Cd treatments was remarkably decreased (Fig. 4c), the carotenoids content in inoculated plants was significantly increased (f = 12.375; p = 0.0055) at all tested Cd treatments (Fig. 4c). Moreover, it was found that the total antioxidant capacity of the inoculated plants was increased by 78.1 and 34% over the non-inoculated plants at 50 and 100 mg L−1 CdCl2 respectively (Fig. 4d).
Enzymatic antioxidants levels
In the current study, the inoculation of safflower with strain EPS significantly (p < 0.05) enhanced the activities of CAT, POX, APX and SOD at all the tested Cd levels (Fig. 5a–d). The inoculation enhanced CAT activity in safflower plants by 15–35%. POX activity upon strain EPS inoculation was found to be increased by 20.6–29.6% under Cd stress compared with the non-inoculated plants. The activities of APX and SOD in safflower plants were improved due to the inoculation by 40.5 to 109.9% and 96.9 to 124.6% over the non-inoculated plants under Cd stress, respectively.
Effect of bacterial inoculation on antioxidant enzyme activities: a catalase (CAT), b guaiacol peroxidase (POX), c ascorbate peroxidase (APX) and d superoxide dismutase (SOD). Values are means ± SEs of three independent replicates (n = 3). Different letter alphabets above the graphs indicate significant differences at p ≤ 0.05 between inoculated and non-inoculated plants according to Tukey's HSD test, while similar letters indicate no significant result
Hydrogen peroxide (H2O2) content in safflower leaves
In the current work, it was found that the inoculation of safflower with strain EPS significantly reduced the accumulation of H2O2 in their leaves under all tested Cd concentrations compared with the non-inoculated plants (Fig. 6).
Effect of bacterial inoculation on hydrogen peroxide (H2O2) content of the leaves under Cd stress. Values are means ± SEs of three independent replicates (n = 3). Different letter alphabets above the graphs indicate significant differences at p ≤ 0.05 between inoculated and non-inoculated plants according to Tukey's HSD test, while similar letters indicate no significant result
Discussion
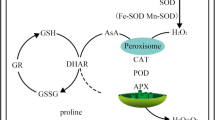
Metal toxicity and stress in plants triggering the excessive accumulation of ROS in mitochondria, chloroplast, and peroxisomes (Kochian et al. 2004), resulting in imbalance between the generation of ROS and antioxidant defense systems, that in turn causes oxidative stress to plants (Gupta et al. 2013). Oxidative stress disturbs physiological and metabolic processes of the plants leading to a limitation in plant growth, crop production and yield, and consequently causes massive agricultural loss (Tran and Popova 2013). Recently, plant root-associated bacteria are globally used for the amelioration of crop performance to encounter heavy metal contamination in agricultural soils (Mitra et al. 2018; He et al. 2020; Ghosh et al. 2022).
Interestingly, the present strain EPS tolerated Cd up to 200 mg L−1 (Fig. 2). Bacteria can tolerate Cd and resist its negative effects using several mechanisms such as transport, precipitation, transformation or intracellular sequestration by thiol containing compounds like metallothionein and glutathione (Intorne et al. 2012; Maynaud et al. 2014). In the present study, strain EPS showed Cd- adsorption ability at both tested Cd concentrations. The ability of strain EPS to adsorb Cd ions may be attributed to the negatively charged functional groups (carboxyl, phosphoryl and hydroxyl) in its polysaccharide structure that can bind the positively charged metal ions. Our finding is in agreement with Liaquat et al. (2020) who reported that Stenotrophomonas maltophilia has remarkable Cd- adsorption potential under varying concentrations.
Levels of antioxidants within the plant cell tend to fluctuate at cadmium exposure (Ali et al. 2019). The interaction between plants and microorganisms at biochemical, physiological and molecular levels largely directs plant responses toward abiotic stresses (Farrar et al. 2014; Meena et al. 2017). This crucial aspect considered as an interest gateway for scientists to search novel cost effective and eco-friendly methods to alleviate the abiotic stresses in field grown plants. The application of bacteria to mitigate stress-induced negative impact in plants and their role to make plants tougher toward abiotic stresses have been documented (Panlada et al. 2013; Nadeem et al. 2014; Kaushal and Wani 2016; Rizvi and Khan 2018; Ghosh et al. 2022). In this study, the efect of S. maltophilia EPS inoculation on the antioxidant defense of safflower plants (Carthamus tinctorius L.) exposed to different levels of Cd was investigated.
The non-enzymatic antioxidants like phenolic compounds, flavonoids, ascorbate as well as carotenoids considered as the half of the antioxidant machinery of the plant cell (Das and Roychoudhury 2014). They play a vital role in the plant cell through protecting the cell components from oxidative damage as well as improving plant growth and development via modifying cellular processes such as mitosis, cell elongation, senescence and cell death (de Pinto and De Gara 2004). Phenolics are better and more efficient antioxidant due to the presence of hydroxyl ions in their structure that can chelate metal ions, trap active oxygen species as well as inhibit lipid peroxidation (Michalak 2006; Ali et al. 2019). Flavonoids are secondary antioxidants with variable phenolic structures that act as reactive oxygen species (ROS) scavengers (Fini et al. 2011; Das and Roychoudhury 2014). In the current study, the inoculation with strain EPS significantly enhanced the total phenolics and total flavonoids contents in safflower plants at all the tested Cd concentrations (Fig. 4a, b).
Carotenoids are lipophilic antioxidants in the plant plastids. They prevent oxidative damage and protect photosynthetic apparatus via detoxifying multiple forms of ROS (McElroy and Kopsell 2009). The exposure to Cd resulted in a decrease of carotenoids contents of safflower plants that attributes to Cd-induced decrease of the photosynthetic rate (Mobin and Khan 2007; Shi et al. 2010). In the present study, the inoculation with strain EPS was significantly improved the quantities of carotenoids antioxidants in safflower plants under Cd stress compared to the non-inoculated plants (Fig. 4c). Moreover, the total antioxidant capacity of safflower plants increased due to bacterial inoculation (Fig. 4d). This is because the total antioxidant capacity was strongly correlated with total phenolics and total flavonoids contents. The positive correlations between total phenolics, total flavonoids and antioxidant activities were reported by other researchers (Gouveia and Castilho 2011; Contreras-Calderón et al. 2011; Aryal et al. 2019; Santos and MagalhÃes 2020; Butkeviciute et al. 2022).
Plants possess multiple antioxidative enzymes including catalase (CAT), guaiacol peroxidase (POX), Ascorbate peroxidase (APX) and superoxide dismutase (SOD) that alleviate oxidative stress and maintain redox homeostasis through catalyting the transformation of ROS into stable nontoxic molecules (Sáez and Están-Capell 2014). In the current study, the inoculation of safflower with strain EPS resulted in significant improvement of the activities of antioxidative enzymes including CAT, POX, APX and SOD (Fig. 5a–d).
As a result of many stresses, the cellular concentration of superoxide radicals increases, which are subsequently converted to hydrogen peroxide by mitochondrial manganese superoxide dismutase (Huseynova et al. 2015). Hydrogen peroxide is one of the major contributors causing oxidative damage to plant cell, leading to inhibition of plant growth and development, or to death (Hung et al. 2005; Hossain et al. 2015). Interestingly, the inoculation with strain EPS led to remarkable reduction in the H2O2 contents of safflower leaves (Fig. 6). This may be because the activity of catalase enzyme increases with increasing Cd level (Fig. 5 a), as an antioxidant defense to breakdown toxic H2O2 into water and divalent oxygen (Cuypers et al. 2010).
Conclusion
An endophytic bacterium Stenotrophomonas maltophilia EPS was isolated from common bean roots. The strain exhibited strong Cd tolerance and adsorption efficiency. The strain was inoculated into safflower seeds to evaluate its effect on plant antioxidant defense under Cd stress. The output of the study is that the inoculation was significantly improved the antioxidant defense of safflower plants under Cd stress through increasing the levels of antioxidant compounds and enhancing the activities of antioxidant enzymes. This study provides an eco-friendly and safety method for alleviating Cd stress in plants, that can guarantee safe agricultural productivity in Cd-contaminated fields.
References
Ali M A, Fahad S, Haider I, Ahmed N, Ahmad S, Hussain S, Arshad M (2019) Oxidative stress and antioxidant defense in plants exposed to metal/metalloid toxicity. In: Reactive oxygen, nitrogen and sulfur species in plants: production, metabolism, signalling and defense mechanisms. Wiley, pp 353– 370. https://doi.org/10.1002/9781119468677.ch15
Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N (2019) Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants (basel, Switzerland) 8(4):96. https://doi.org/10.3390/plants8040096
Asgarpanah J, Kazemivash N (2013) Phytochemistry, pharmacology and medicinal properties of Carthamus tinctorius L. Chin J Integr Med 19(2):153–159. https://doi.org/10.1007/s11655-013-1354-5
Bernard A (2008) Cadmium and its adverse effects on human health. Indian J Med Res 128(4):557 (PMID: 19106447)
Boyajian GE, Carreira LH (1997) Phytoremediation: A clean transition from laboratory to marketplace? Nat Biotechnol 15(2):127–128. https://doi.org/10.1038/nbt0297-127
Butkeviciute A, Abukauskas V, Janulis V, Kviklys D (2022) Phenolic content and antioxidant activity in apples of the ‘Galaval’ cultivar grown on 17 different rootstocks. Antioxidants 11(2):266. https://doi.org/10.3390/antiox11020266
Cavalcanti FR, Oliveira JTA, Martins-Miranda AS, Viégas RA, Silveira JAG (2004) Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytol 163:563–571. https://doi.org/10.1111/j.1469-8137.2004.01139.x
Chang C, Yang M, Wen H, Chern J (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10:178–182. https://doi.org/10.38212/2224-6614.2748
Contreras-Calderón J, Calderón-Jaimes L, Guerra-Hernández E, García-Villanova B (2011) Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Res Int 44(7):2047–2053. https://doi.org/10.1016/j.foodres.2010.11.003
Cuypers A, Plusquin M, Remans T, Jozefczak M, Smeets K (2010) Cadmium stress: an oxidative challenge. Biometals 23(5):927–940. https://doi.org/10.1007/s10534-010-9329-x
Dai XP, Feng L, Ma XW, Zhang YM (2012) Concentration level of heavy metals in wheat grains and the health risk assessment to local inhabitants from Baiyin, Gansu, China. Adv Mat Res 518:951–956. https://doi.org/10.4028/www.scientific.net/AMR.518-523.951
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2:53. https://doi.org/10.3389/fenvs.2014.00053
de Pinto MC, De Gara L (2004) Changes in the ascorbate metabolism of apoplastic and symplastic spaces are associated with cell differentiation. J Exp Bot 55:2559–2569. https://doi.org/10.1093/jxb/erh253
Delshad E, Yousefi M, Sasannezhad P, Rakhshandeh H, Ayati Z (2018) Medical uses of Carthamus tinctorius L. (Safflower): a comprehensive review from Traditional Medicine to Modern Medicine. Electron Physician 10(4):6672–6681. https://doi.org/10.19082/6672
Dordas CA, Sioulas C (2008) Safflower yield, chlorophyll content, photosynthesis, and water use efficiency response to nitrogen fertilization under rainfed conditions. Ind Crops Prod 27(1):75–85. https://doi.org/10.1016/j.indcrop.2007.07.020
Du H, Chen W, Cai P, Rong X, Chen CR, Huang Q (2016) Cadmium adsorption on bacteria–mineral mixtures: effect of naturally occurring ligands. Eur J Soil Sci 67:641–649. https://doi.org/10.1111/ejss.12373
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356. https://doi.org/10.1021/ac60111a017
Farrar K, Bryant D, Cope-Selby N (2014) Understanding and engineering beneficial plant–microbe interactions: plant growth promotion in energy crops. Plant Biotechnol J 12:1193–1206. https://doi.org/10.1111/pbi.12279
Fini A, Brunetti C, Di Ferdinando M, Ferrini F, Tattini M (2011) Stress-induced flavonoid biosynthesis and the antioxidant machinery of plants. Plant Signal Behav 6:709–711. https://doi.org/10.4161/psb.6.5.15069
Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ (2008) Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol 74:2461–2470. https://doi.org/10.1128/AEM.02272-07
Fryzova R, Pohanka M, Martinkova P, Cihlarova H, Brtnicky M, Hladky J, Kynicky J (2017) Oxidative stress and heavy metals in plants. In: de Voogt P (ed) Reviews of environmental contamination and toxicology Volume 245. Reviews of Environmental Contamination and Toxicology (Continuation of Residue Reviews), vol 245. Springer, Cham. https://doi.org/10.1007/398_2017_7
Ghosh A, Pramanik K, Bhattacharya S, Mondal S, Ghosh SK, Maiti TK (2022) A potent cadmium bioaccumulating Enterobacter cloacae strain displays phytobeneficial property in Cd-exposed rice seedlings. CRMICR 3:100101. https://doi.org/10.1016/j.crmicr.2021.100101
Gill SS, Hasanuzzaman M, Nahar K, Macovei A, Tuteja N (2013) Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol Biochem 63:254–261. https://doi.org/10.1016/j.plaphy.2012.12.001
Gouveia S, Castilho PC (2011) Antioxidant potential of Artemisia argentea L’ alcoholic extract and its relation with the phenolic composition. Food Res Int 44(6):1620–1631. https://doi.org/10.1016/j.foodres.2011.04.040
Gupta N, Gaurav SS, Kumar A (2013) Molecular basis of aluminium toxicity in plants: a review. Am J Plant Sci 4:21–37. https://doi.org/10.4236/ajps.2013.412A3004
He S, He Z, Yang X, Stoffella PJ, Baligar VC (2015) Soil biogeochemistry, plant physiology, and phytoremediation of cadmium-contaminated soils. Adv Agron 134:135–225. https://doi.org/10.1016/bs.agron.2015.06.005
He X, Xu M, Wei Q, Tang M, Guan L, Lou L et al (2020) Promotion of growth and phytoextraction of cadmium and lead in Solanum nigrum L. mediated by plant growth-promoting rhizobacteria. Ecotoxicol Environ Saf 205:111333. https://doi.org/10.1016/j.ecoenv.2020.111333
Hossain MA, Bhattacharjee S, Armin SM, Qian P, Xin W, Li HY, Burritt DJ, Fujita M, Tran LS (2015) Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: insights from ROS detoxification and scavenging. Front Plant Sci 6:420. https://doi.org/10.3389/fpls.2015.00420
Hung S-H, Yu C-W, Lin CH (2005) Hydrogen peroxide functions as a stress signal in plants. Bot Bull Acad Sin 46:1–10
Huseynova IM, Aliyeva DR, Mammadov AC, Aliyev JA (2015) Hydrogen peroxide generation and antioxidant enzyme activities in the leaves and roots of wheat cultivars subjected to long-term soil drought stress. Photosynth Res 125:279–289. https://doi.org/10.1007/s11120-015-0160-7
Intorne AC, De Oliveira MVV, De Pereira ML, De Souza FGA (2012) Essential role of the czc determinant for cadmium, cobalt and zinc resistance in Gluconacetobacter diazotrophicus PAl 5. Int Microbiol 15(2):69–78. https://doi.org/10.2436/20.1501.01.160
Kato M, Shimizu S (1987) Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves; phenolic-dependent peroxidative degradation. Can J Bot 65(4):729–735. https://doi.org/10.1139/b87-097
Kaushal M, Wani SP (2016) Plant-growth-promoting rhizobacteria: drought stress alleviators to ameliorate crop production in drylands. Ann Microbiol 66:35–42. https://doi.org/10.1007/s13213-015-1112-3
Kim Y, Yoo JY (1996) Peroxidase production from carrot hairy root cell culture. Enzyme Microb Technol 18(7):531–535. https://doi.org/10.1016/0141-0229(95)00168-9
Kochian LV, Hoekenga OA, Pineros MA (2004) How do crop plants tolerate acid soils? Mechanisms of Al tolerance and phosphorous efficiency. Annu Rev Plant Biol 55:459–493. https://doi.org/10.1146/annurev.arplant.55.031903.141655
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35(6):1547–1549. https://doi.org/10.1093/molbev/msy096
Liaquat F, Munis M, Arif S, Haroon U, Shengquan C, Qunlu L (2020) Cd-tolerant SY-2 strain of Stenotrophomonas maltophilia: a potential PGPR, isolated from the Nanjing mining area in China. 3 Biotech 10(12):519. https://doi.org/10.1007/s13205-020-02524-7
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592. https://doi.org/10.1042/bst0110591
Ma Y, Prasad MNV, Rajkumar M, Freitas H (2011) Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol Adv 29(2):248–258. https://doi.org/10.1016/j.biotechadv.2010.12.001
Ma Y, Oliveira RS, Freitas H, Zhang C (2016) Biochemical and molecular mechanisms of plant-microbe-metal interactions: relevance for phytoremediation. Front Plant Sci 7:918. https://doi.org/10.3389/fpls.2016.00918
Maynaud G, Brunel B, Yashiro E, Mergeay M, Cleyet-Marel JC, Le Quere A (2014) CadA of Mesorhizobium metallidurans isolated from a zinc-rich mining soil is a P(IB-2)-type ATPase involved in cadmium and zinc resistance. Res Microbiol 165:175–189. https://doi.org/10.1016/j.resmic.2014.02.001
McElroy JS, Kopsell DA (2009) Physiological role of carotenoids and other antioxidants in plants and application to turfgrass stress management. N Z J Crop Hortic Sci 37(4):327–333. https://doi.org/10.1080/01140671.2009.9687587
Meagher RB (2000) Phytoremediation of toxic elemental and organic pollutants. Curr Opin Plant Biol 3(2):153–162. https://doi.org/10.1016/s1369-5266(99)00054-0
Meena KK, Sorty AM, Bitla UM, Choudhary K, Gupta P, Pareek A, Singh DP, Prabha R, Sahu PK, Gupta VK, Singh HB, Krishanani KK, Minhas PS (2017) Abiotic stress responses and microbe-mediated mitigation in plants: the omics strategies. Front Plant Sci 8:172. https://doi.org/10.3389/fpls.2017.00172
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud 15(4):523–530
Mitra S, Pramanik K, Sarkar A, Ghosh PK, Soren T, Maiti TK (2018) Bioaccumulation of cadmium by Enterobacter sp. and enhancement of rice seedling growth under cadmium stress. Ecotoxicol Environ Saf 156:183–196. https://doi.org/10.1016/j.ecoenv.2018.03.001
Mobin M, Khan NA (2007) Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol 164(5):601–610. https://doi.org/10.1016/j.jplph.2006.03.003
Nadeem SM, Ahmad M, Zahir ZA, Javaid A, Ashraf M (2014) The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol Adv 32:429–448. https://doi.org/10.1016/j.biotechadv.2013.12.005
Namdjoyan SH, Khavari-Nejad RA, Bernard F, Nejadsattari T, Shaker H (2011) Antioxidant defense mechanisms in response to cadmium treatments in two safflower cultivars. Russ J Plant Physiol 58:467–477. https://doi.org/10.1134/S1021443711030149
Panlada T, Pongdet P, Aphakorn L, Rujirek NN, Nantakorn B, Neung T (2013) Alleviation of the effect of environmental stresses using co-inoculation of mungbean by Bradyrhizobium and rhizobacteria containing stress-induced ACC deaminase enzyme. Soil Sci Plant Nut 59:559–571. https://doi.org/10.1080/00380768.2013.804391
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem 269(2):337–341. https://doi.org/10.1006/abio.1999.4019
Raja V, Majeed U, Kang H, Andrabi KI, John R (2017) Abiotic stress: Interplay between ROS, hormones, and MAPKs. Environ Exp Bot 137:142–157. https://doi.org/10.1016/j.envexpbot.2017.02.010
Rizvi A, Khan MS (2018) Heavy metal induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen fixing Azotobacter chroococcum. Ecotoxicol Environ Saf 157:9–20. https://doi.org/10.1016/j.ecoenv.2018.03.063
Rizwan M, Ali S, Adrees M, Rizvi H, Rehman MZ, Hannan F, Qayyum MF, Hafeez F, Ok YS (2016) Cadmium stress in rice: toxic effects, tolerance mechanisms and management: a critical review. Environ Sci Pollut Res 23(18):17859–17879. https://doi.org/10.1007/s11356-016-6436-4
Romero-Puertas MC, Rodríguez-Serrano M, Corpas FJ, Gomez MD, Del Rio LA, Sandalio LM (2004) Cadmium-induced subcellular accumulation of O2 and H2O2 in pea leaves. Plant Cell Environ 27(9):1122–1134. https://doi.org/10.1111/j.1365-3040.2004.01217.x
Sáez GT, Están-Capell N (2014) Antioxidant enzymes. In: Schwab M (ed) Encyclopedia of cancer. Springer, Berlin. https://doi.org/10.1007/978-3-662-46875-3_7210
Santos WNLD, MagalhÃes BEA (2020) Phenolic content and antioxidant capacity of infusions herbs: Optimization of phenolic extraction and HPLC-DAD method. An Acad Bras Cienc 92(3):e20190646. https://doi.org/10.1590/0001-3765202020190646
Senthilkumar M, Amaresan N, Sankaranarayanan A (2021) Estimation of ascorbate peroxidase (APX). In: Plant-microbe interactions. Springer Protocols Handbooks. Humana, New York, https://doi.org/10.1007/978-1-0716-1080-0_30
Shi G, Liu C, Cai Q, Liu Q, Hou C (2010) Cadmium accumulation and tolerance of two safflower cultivars in relation to photosynthesis and antioxidative enzymes. Bull Environ Contam Toxicol 85(3):256–263. https://doi.org/10.1007/s00128-010-0067-0
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In: Packer L (ed) Methods in enzymology: oxidants and antioxidants Part A, vol 299. Academic Press, London, pp 152–178. https://doi.org/10.1016/s0076-6879(99)99017-1
Sytar O, Kumar A, Latowski D, Kuczynska P, Strzałka K, Prasad MNV (2013) Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants. Acta Physiol Plant 35(4):985–999. https://doi.org/10.1007/s11738-012-1169-6
Tirry N, Tahri Joutey N, Sayel H, Kouchou A, Bahafid W, Asri M et al (2018) Screening of plant growth promoting traits in heavy metals resistant bacteria: prospects in phytoremediation. J Genet Eng Biotechnol 16(2):613–619. https://doi.org/10.1016/j.jgeb.2018.06.004
Tran TA, Popova LP (2013) Functions and toxicity of cadmium in plants: recent advances and future prospects. Tur J Bot 37(1):1–13. https://doi.org/10.3906/bot-1112-16
Van Rossun MWPC, Alberda M, Van Der Plas LHW (1997) Role of oxidative damage in tulip bulb scale micropropagation. Plant Sci 130(2):207–216. https://doi.org/10.1016/S0168-9452(97)00215-X
Vashishth A, Khanna S (2015) Toxic heavy metals tolerance in bacterial isolates based on their inducible mechanism. Int J Novel Res Life Sci 2:34–41. https://doi.org/10.21776/ub.rjls.2015.002.01.5
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain treated bean plants, protective role of exogenous polyamines. Plant Sci 151(1):59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
Vincent JM (1970) "A Manual for the Practical Study of the Root-nodule Bacteria.” IBP15. Blackwell Scientific Publications, Oxford
Wasay SA, Barrington SF, Tokunaga S (1998) Using Aspergillus niger to bioremediate soils contaminated by heavy metals. Bioremediat J 2(3–4):183–190
Weiss E (2000) Oilseed crops, second addition. Blackwell Science, London
Zengin FK, Munzuroglu O (2005) Effects of some heavy metals on content of chlorophyll, proline and some antioxidant chemicals in bean (Phaseolus vulgaris L.) seedlings. Acta Biol Crac Ser Bot 47(2):157–164
Acknowledgements
We introduce our sincere thanks and gratitude to the Botany Department, Faculty of Science, Aswan University for supporting and providing the requirements of scientific research.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
N.Sh.A.H. contributed to the study design. Material preparation, Methodology, data collection and analysis, and wrote the main manuscript text, and U.M.A-R. contributed to data analysis, read, and reviewed the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hagaggi, N.S.A., Abdul-Raouf, U.M. The endophyte Stenotrophomonas maltophilia EPS modulates endogenous antioxidant defense in safflower (Carthamus tinctorius L.) under cadmium stress. Arch Microbiol 204, 431 (2022). https://doi.org/10.1007/s00203-022-03049-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03049-8