Abstract
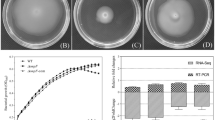
Shewanella oneidensis is a Gram-negative facultative anaerobe that can use a wide variety of terminal electron acceptors for anaerobic respiration. In this study, S. oneidensis degQ gene, encoding a putative periplasmic serine protease, was cloned and expressed. The activity of purified DegQ was inhibited by diisopropyl fluorophosphate, a typical serine protease-specific inhibitor, indicating that DegQ is a serine protease. In-frame deletion and subsequent complementation of the degQ were carried out to examine the effect of envelope stress on the production of outer membrane vesicles (OMVs). Analysis of periplasmic proteins from the resulting S. oneidensis strain showed that deletion of degQ induced protein accumulation and resulted in a significant decrease in protease activity within the periplasmic space. OMVs from the wild-type and mutant strains were purified and observed by transmission electron microscopy. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the OMVs showed a prominent band at ~37 kDa. Nanoliquid chromatography–tandem mass spectrometry analysis identified three outer membrane porins (SO3896, SO1821, and SO3545) as dominant components of the band, suggesting that these proteins could be used as indices for comparing OMV production by S. oneidensis strains. Quantitative evaluation showed that degQ-deficient cells had a fivefold increase in OMV production compared with wild-type cells. Thus, the increased OMV production following the deletion of DegQ in S. oneidensis may be responsible for the increase in envelope stress.




Similar content being viewed by others
References
Belchik SM, Kennedy DW, Dohnalkova AC, Wang Y, Sevinc PC, Wu H, Lin Y, Lu HP, Fredrickson JK, Shi L (2011) Extracellular reduction of hexavalent chromium by cytochromes MtrC and OmcA of Shewanella oneidensis MR-1. Appl Environ Microbiol 77:4035–4041. doi:10.1128/aem.02463-10
Beveridge TJ (1999) Structures of gram-negative cell walls and their derived membrane vesicles. J Bacteriol 181:4725–4733
Dai J, Wei H, Tian C, Damron FH, Zhou J, Qiu D (2015) An extracytoplasmic function sigma factor-dependent periplasmic glutathione peroxidase is involved in oxidative stress response of Shewanella oneidensis. BMC Microbiol 15:34. doi:10.1186/s12866-015-0357-0
Edwards RA, Keller LH, Schifferli DM (1998) Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207:149–157
Fennessey CM, Jones ME, Taillefert M, DiChristina TJ (2010) Siderophores are not involved in Fe(III) solubilization during anaerobic Fe(III) respiration by Shewanella oneidensis MR-1. Appl Environ Microbiol 76:2425–2432. doi:10.1128/aem.03066-09
Fredrickson JK, Romine MF, Beliaev AS, Auchtung JM, Driscoll ME, Gardner TS, Nealson KH, Osterman AL, Pinchuk G, Reed JL, Rodionov DA, Rodrigues JL, Saffarini DA, Serres MH, Spormann AM, Zhulin IB, Tiedje JM (2008) Towards environmental systems biology of Shewanella. Nat Rev Microbiol 6:592–603. doi:10.1038/nrmicro1947
Gao H, Barua S, Liang Y, Wu L, Dong Y, Reed S, Chen J, Culley D, Kennedy D, Yang Y, He Z, Nealson KH, Fredrickson JK, Tiedje JM, Romine M, Zhou J (2010) Impacts of Shewanella oneidensis c-type cytochromes on aerobic and anaerobic respiration. Microb Biotechnol 3:455–466. doi:10.1111/j.1751-7915.2010.00181.x
Gao T, Ju L, Yin J, Gao H (2015) Positive regulation of the Shewanella oneidensis OmpS38, a major porin facilitating anaerobic respiration, by Crp and Fur. Sci Rep 5:14263. doi:10.1038/srep14263
Gujrati V, Kim S, Kim SH, Min JJ, Choy HE, Kim SC, Jon S (2014) Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano 8:1525–1537. doi:10.1021/nn405724x
Heidelberg JF, Paulsen IT, Nelson KE, Gaidos EJ, Nelson WC, Read TD, Eisen JA, Seshadri R, Ward N, Methe B, Clayton RA, Meyer T, Tsapin A, Scott J, Beanan M, Brinkac L, Daugherty S, DeBoy RT, Dodson RJ, Durkin AS, Haft DH, Kolonay JF, Madupu R, Peterson JD, Umayam LA, White O, Wolf AM, Vamathevan J, Weidman J, Impraim M, Lee K, Berry K, Lee C, Mueller J, Khouri H, Gill J, Utterback TR, McDonald LA, Feldblyum TV, Smith HO, Venter JC, Nealson KH, Fraser CM (2002) Genome sequence of the dissimilatory metal ion-reducing bacterium Shewanella oneidensis. Nat Biotechnol 20:1118–1123. doi:10.1038/nbt749
Kadurugamuwa JL, Beveridge TJ (1995) Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol 177:3998–4008
Kadurugamuwa JL, Beveridge TJ (1996) Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J Bacteriol 178:2767–2774
Knox KW, Vesk M, Work E (1966) Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J Bacteriol 92:1206–1217
Lee EY, Bang JY, Park GW, Choi DS, Kang JS, Kim HJ, Park KS, Lee JO, Kim YK, Kwon KH, Kim KP, Gho YS (2007) Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics 7:3143–3153. doi:10.1002/pmic.200700196
Lipinska B, Zylicz M, Georgopoulos C (1990) The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J Bacteriol 172:1791–1797
Maier TM, Myers CR (2004) The outer membrane protein Omp35 affects the reduction of Fe(III), nitrate, and fumarate by Shewanella oneidensis MR-1. BMC Microbiol 4:23. doi:10.1186/1471-2180-4-23
McBroom AJ, Kuehn MJ (2007) Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol Microbiol 63:545–558. doi:10.1111/j.1365-2958.2006.05522.x
McBroom AJ, Johnson AP, Vemulapalli S, Kuehn MJ (2006) Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J Bacteriol 188:5385–5392. doi:10.1128/JB.00498-06
Miller VL, Mekalanos JJ (1988) A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol 170:2575–2583
Myers CR, Myers JM (1994) Ferric iron reduction-linked growth yields of Shewanella putrefaciens MR-1. J Appl Bacteriol 76:253–258
Myers JM, Myers CR (2000) Role of the tetraheme cytochrome CymA in anaerobic electron transport in cells of Shewanella putrefaciens MR-1 with normal levels of menaquinone. J Bacteriol 182:67–75
Myers CR, Nealson KH (1988) Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319–1321. doi:10.1126/science.240.4857.1319
Myers CR, Nealson KH (1990) Respiration-linked proton translocation coupled to anaerobic reduction of manganese(IV) and iron(III) in Shewanella putrefaciens MR-1. J Bacteriol 172:6232–6238
Nguyen MH, Ojima Y, Sakka M, Sakka K, Taya M (2014) Probing of exopolysaccharides with green fluorescence protein-labeled carbohydrate-binding module in Escherichia coli biofilms and flocs induced by bcsB overexpression. J Biosci Bioeng 118:400–405. doi:10.1016/j.jbiosc.2014.03.005
Nowotny A, Behling UH, Hammond B, Lai CH, Listgarten M, Pham PH, Sanavi F (1982) Release of toxic microvesicles by Actinobacillus actinomycetemcomitans. Infect Immun 37:151–154
Penfold RJ, Pemberton JM (1992) An improved suicide vector for construction of chromosomal insertion mutations in bacteria. Gene 118:145–146
Quan S, Hiniker A, Collet J-F, Bardwell CAJ (2013) Isolation of bacteria envelope proteins. In: Delcour AH (ed) Bacterial cell surfaces: methods and protocols. Springer, Berlin, pp 359–366
Rothfield L, Pearlman-Kothencz M (1969) Synthesis and assembly of bacterial membrane components. A lipopolysaccharide–phospholipid–protein complex excreted by living bacteria. J Mol Biol 44:477–492
Schwechheimer C, Kuehn MJ (2013) Synthetic effect between envelope stress and lack of outer membrane vesicle production in Escherichia coli. J Bacteriol 195:4161–4173. doi:10.1128/jb.02192-12
Schwechheimer C, Kuehn MJ (2015) Outer-membrane vesicles from Gram-negative bacteria: biogenesis and functions. Nat Rev Microbiol 13:605–619. doi:10.1038/nrmicro3525
Schwechheimer C, Sullivan CJ, Kuehn MJ (2013) Envelope control of outer membrane vesicle production in Gram-negative bacteria. Biochemistry 52:3031–3040. doi:10.1021/bi400164t
Secades P, Guijarro JA (1999) Purification and characterization of an extracellular protease from the fish pathogen Yersinia ruckeri and effect of culture conditions on production. Appl Environ Microbiol 65:3969–3975
Strauch KL, Johnson K, Beckwith J (1989) Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol 171:2689–2696
Sundararajan A, Kurowski J, Yan T, Klingeman DM, Joachimiak MP, Zhou J, Naranjo B, Gralnick JA, Fields MW (2011) Shewanella oneidensis MR-1 sensory box protein involved in aerobic and anoxic growth. Appl Environ Microbiol 77:4647–4656. doi:10.1128/aem.03003-10
Swamy KH, Chung CH, Goldberg AL (1983) Isolation and characterization of protease do from Escherichia coli, a large serine protease containing multiple subunits. Arch Biochem Biophys 224:543–554
Takeshita S, Sato M, Toba M, Masahashi W, Hashimoto-Gotoh T (1987) High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene 61:63–74
Wensink J, Witholt B (1981) Identification of different forms of the murein-bound lipoprotein found in isolated outer membranes of Escherichia coli. Eur J Biochem 113:349–357
Zhou L, Srisatjaluk R, Justus DE, Doyle RJ (1998) On the origin of membrane vesicles in Gram-negative bacteria. FEMS Microbiol Lett 163:223–228. doi:10.1111/j.1574-6968.1998.tb13049.x
Acknowledgements
This study was supported in part by a grant from the Noda Institute for Scientific Research. We thank Dr. Muranaka at the Research Center for Ultra-High Voltage Electron Microscopy, Osaka University, Japan, for assistance with TEM imaging.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Electrophoresis of the degQ fragment amplified from genomic DNA isolated from S. oneidensis wild-type (lane 1) and ΔdegQ (lane 2) strains. Lane M; HindIII-digested λ DNA marker (TIFF 1036 kb)
Rights and permissions
About this article
Cite this article
Ojima, Y., Mohanadas, T., Kitamura, K. et al. Deletion of degQ gene enhances outer membrane vesicle production of Shewanella oneidensis cells. Arch Microbiol 199, 415–423 (2017). https://doi.org/10.1007/s00203-016-1315-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-016-1315-4