Abstract
Summary
The use of buffered soluble alendronate 70 mg effervescent tablet, a convenient dosing regimen for bisphosphonate therapy, seems a cost-effective strategy compared with relevant alternative treatments for postmenopausal women with osteoporosis aged 60 years and over in Italy.
Introduction
To assess the cost-effectiveness of buffered soluble alendronate (ALN) 70 mg effervescent tablet compared with relevant alternative treatments for postmenopausal osteoporotic women in Italy.
Methods
A previously validated Markov microsimulation model was adjusted to the Italian healthcare setting to estimate the lifetime costs (expressed in €2019) per quality-adjusted life-years (QALY) of buffered soluble ALN compared with generic ALN, denosumab, zoledronic acid and no treatment. Pooled efficacy data derived from the NICE network meta-analysis were used for bisphosphonate treatments. Two treatment duration scenarios were assessed: 1 year using persistence data derived from an Italian prospective observational study including 144 and 216 postmenopausal osteoporotic women on buffered soluble ALN and oral ALN, respectively, and 3 years. Analyses were conducted for women 60–80 years of age with a bone mineral density T-score ≤ − 3.0 or with existing vertebral fractures.
Results
In all simulated populations, buffered soluble ALN was dominant (more QALYs, lower costs) compared to denosumab. The cost per QALY gained of buffered soluble ALN compared to generic ALN and no treatment always falls below €20,000 per QALY gained. In the 1-year treatment scenario, zoledronic acid was associated with more QALY than buffered soluble ALN but the cost per QALY gained of zoledronic acid compared with buffered soluble ALN was always higher than €70,000, while buffered soluble ALN was dominant in the 3-year treatment scenario.
Conclusion
This study suggests that buffered soluble ALN represents a cost-effective strategy compared with relevant alternative treatments for postmenopausal osteoporosis women in Italy aged 60 years and over.
Similar content being viewed by others
Introduction
Osteoporosis and related osteoporotic fractures represent a major health problem worldwide and are on the rise. A recent study suggested that the total fragility fractures in the largest five EU countries (UK, Italy, France, Germany and Spain) and Sweden were estimated to 2.7 million in 2017, resulting in annual fracture-related costs of €37.5 billion. The number of fragility fractures and total costs were further estimated to increase by 23% and 27%, respectively, by the year 2030. In addition, the treatment gap (defined as the percentage of eligible individuals not receiving treatment with osteoporosis drugs) was estimated to be 73% for women, an increase of 17% since 2010 [1].
Oral bisphosphonates (including alendronate (ALN) and risedronate) remain the most widely used treatment of osteoporosis [2]. Poor adherence (defined as the process by which patients take their medication as prescribed, further divided into three quantifiable phases: ‘initiation’, ‘implementation’ and ‘discontinuation’ [3]) to oral bisphosphonates is however a major concern [4], suggesting the need for medications more cost-effective and more likely to be taken. Approximately 50% of women who initiate oral bisphosphonates discontinue therapy within 1 year, and patients on oral bisphosphonates frequently miss doses and therefore do not implement treatment as prescribed [5]. Different barriers to osteoporosis medication adherence have been identified [6], including treatment-related factors such as medication side effects, complex instructions for medication administration and complex medication regimens [4, 6]. Oral bisphosphonates require patients to follow strict dosing instructions to derive the full benefits, i.e. the intake on an empty stomach at least 30 to 60 min before the first food, drink or other medication of the day [7].
Recently, buffered soluble ALN 70 mg (Binosto®) was developed with the aim to improve the gastro-intestinal (GI) tolerability through full dissolution of ALN in buffered palatable solution before ingestion to facilitate passage of the buffered ALN solution into the stomach, minimize the contact of solid ALN particles with the GI mucosa and buffer the stomach acid with its pH of 4.8–5.4 and thus minimize the risk of GI irritation [8]. Research has confirmed that buffered soluble ALN leads to a lower frequency of GI adverse reactions [9] that could lead to improved adherence.
Considering the limited healthcare resources available, it is important to assess whether buffered soluble ALN represents good value for money compared with relevant alternative treatments. Cost-effectiveness analyses that compare interventions in terms of costs and outcomes are nowadays increasingly important and used by decision makers to efficiently allocate scarce healthcare resources, especially for pricing and reimbursement decisions. With the increasing burden of osteoporosis and development of pharmacological options, numerous cost-effectiveness studies have been conducted in last decades to assess the cost-effectiveness of osteoporosis drugs [10]. However, to our knowledge, no study has yet estimated the cost-effectiveness of buffered soluble ALN, an alendronate effervescent tablet.
The aim of this study was therefore to assess the cost-effectiveness of buffered soluble ALN compared with no treatment, generic ALN, denosumab and zoledronic acid for the treatment of postmenopausal women with osteoporosis in Italy.
Methods
The current study followed the recent ESCEO-IOF recommendations for the conduct and reporting of economic evaluations in osteoporosis [11] and adhered to the Consolidated Health Economic Evaluation Reporting Standards statement [12]. A Markov microsimulation model was previously built and validated to assess the cost-effectiveness of osteoporosis management in several countries [13,14,15,16]. The most recent version of the model was adapted to the Italian healthcare context to estimate the cost-effectiveness of buffered soluble ALN compared with no treatment, generic alendronate, denosumab and zoledronic acid. The model simulated the entire lifetime of women (up to 100 years old or until death) to capture relevant costs and health consequences of fracture events. The model was built up using TreeAge Pro 2020 (TreeAge Pro Inc., Williamston, MA, USA). A description of the model is provided here below, key model parameters are included in Table 1 and additional information could be found in previous publication [15], including an online appendix providing a very detailed explanation of the model [14]. A list of key model components/assumptions is further provided in Appendix 1 Table 1.
Model structure
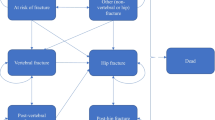
A Markov microsimulation model with a 6-month cycle length was used to allow tracking patient characteristics and individual disease histories (e.g. fractures) and avoid unnecessary transition restrictions. The model health states were ‘no fracture’ (where all individuals begin), ‘death’, ‘hip fracture’, ‘clinical vertebral fracture’, ‘wrist fracture’ and ‘other fracture’. The ‘other fracture’ state includes other osteoporotic fractures as defined by the IOF-EFPIA report [17]. Patients could experience multiple fractures at the same site or multiple sites. Discount rates of 3% for both costs and health benefits were used in line with the Italian guideline for economic evaluations.
Populations
Analyses were conducted at different age (60–80 years) in two populations with high risk of fragility fractures in line with reimbursement conditions in Italy (Nota 79): (a) in postmenopausal female patients with existing vertebral fracture, and (b) those with osteoporosis as defined by T-score ≤ − 3.0 at the femoral neck and without vertebral fracture.
Fracture risk
Information concerning all hospitalizations occurring in Italy is registered in hospital discharge records, which are collected at central level by the Italian Ministry of Health (National Hospitalization Database). The incidence of hip fractures in the general Italian population was derived from this database for the year 2017 for different age groups (65–69, 70–74, 75–79, 80–84, 85–89, 90–94 and 95+ years) [18]. As these recent data did not include women aged lower than 65 years, the ratio of hip fractures between 60–64 and 65–69 years from Piscitelli et al. [19] for the year 2008 was applied to derive incidence rate for the age group 60–64 years. To estimate the incidence of clinical vertebral and wrist fractures, ratios between hip fracture and clinical vertebral/wrist fractures from an older Italian database from the year 2008 were applied [20]. As no data for other fractures are available at national level in Italy, we applied the age-specific ratio incidence from other countries in line with the methodology used by the IOF-EFPIA report [17].
Initial probabilities were then adjusted to accurately reflect the fracture risk in the target population in comparison with that of the general population using previously validated methods [21, 22]. The methods calculated the relative risk of individuals below the threshold value (i.e. BMD T-score ≤ − 3.0) and of individuals with prevalent vertebral fractures compared with that of the general population. Fracture risk was also adjusted when a new fracture occurred during the simulation process in line with studies suggesting an increased risk for fracture after a prior fracture [21].
The age-specific mortality rates for the general population were derived from the Italian Institute of Statistics for the year 2018. An increased mortality after hip fracture and clinical vertebral fracture was modelled in line with previous studies [23]. Because excess mortality may also be attributable to comorbidities, we further took into account that only 25% of the excess mortality following fractures was attributable to the fractures themselves [24, 25].
Fracture cost
The healthcare decision maker perspective was used for the cost estimation. All costs were expressed in €2019 and adjusted using the national price index. The cost of hip fractures was derived from the study of Piscitelli et al. with data from the year 2014 [26]. In the absence of local data, the costs of non-hip fractures were quantified relative to hip fracture in line with the assumption used in the IOF-EFPIA report [17]. This assumption is conservative compared to a previous cost-effectiveness study conducted in Italy [27]. Long-term hip fracture costs were based on the proportion of patients being institutionalized following the hip fracture, estimated at 10% in Italy [19, 26], while the annual cost of being in the nursing home was estimated at €41,300 [28].
Utility values
Utility values were derived from the International Costs and Utilities Related to Osteoporotic Fractures Study (ICUROS) study [29]. This study is the largest study assessing the quality of life of patients with fractures from 11 countries including 2,808 patients, and collected EQ-5D data at different time points after fractures and a recall before fractures. Baseline utility data (for patients without fractures) were derived from the utility estimation before fractures. Decreases in quality of life as a consequence of fracture were included as utility multipliers for the first year following fracture and in subsequent years. Since other fractures were not included in the ICUROS study, estimate from a previous systematic review was used [30]. An additional effect on utility after multiple fractures was modelled [13, 14].
Treatments
In base case, the effects of treatment on the risk of fractures (expressed as relative risks, RR) were derived from the most recent network meta-analysis of the National Institute for Health and Care Excellence (NICE) in the UK (ta464) [31]. In the absence of studies suggesting a clear and significant difference between bisphosphonates, and in line with the NICE committee discussion regarding bisphosphonates (appraisal TA464) suggesting that the efficacy estimates of the oral and intravenous bisphosphonates should be pooled for each fracture site, results of the network meta-analysis of pooled bisphosphonates data were used for all bisphosphonates including buffered soluble ALN, generic ALN and zoledronic acid. After stopping medication, it was assumed a linear decrease of the effects for a duration similar to the duration of therapy, in line with previous economic analyses of oral bisphosphonates [10] and clinical data [32]. The effect of denosumab on fracture risk was derived from the FREEDOM trial [33]. In line with recent evidence suggesting an immediate bone loss after denosumab discontinuation [34], an immediate loss of treatment effect following treatment discontinuation was assumed.
Two treatment duration scenarios (1 and 3 years) were assessed and adjusted by medication persistence using a previously used methodology [35, 36]. Medication persistence was measured as the probability to be on treatment at different time points, and has been shown to be the driver of adherence in economic evaluations in osteoporosis [37]. In the first scenario, treatment duration was set-up to 1 year in line with persistence data available for buffered soluble ALN derived from an Italian prospective observational study [38] (see later). In line with previous literature [39,40,41], it was assumed that 90% and 80% of patients under denosumab are persistent at 6 and 12 months respectively. For patients who discontinued therapy, treatment cost was stopped and the offset time period started immediately. For those who discontinued oral/effervescent bisphosphonates within 6 months, no treatment effect was received, because at least 6 months of treatment is necessary to reduce the risk of fractures. In the second scenario, a maximum of 3-year treatment duration was assumed for all treatments to better reflect clinical practices. Real-world persistence data with oral bisphosphonates were derived from a recent systematic review suggesting that the mean persistence was 53% at 6 months, 46% at 1 year, 37% at 2 years and 31% at 3 years [42]. Another systematic review of articles published up to September 2018 identified ten studies reporting zoledronic acid persistence rates with mean persistence rates of 52% and 36% for second and third dose, respectively [41]. Denosumab was reported in 19 studies, with mean persistence rates of 81%, 55% and 35% at second (year 1), fourth and fifth doses (year 2 and year 2.5) [41]. The (relative) positive effect of buffered soluble ALN on persistence at year 1 compared to oral ALN was assumed to be maintained up to 3 years, leading to persistence rates for buffered soluble ALN of 83% at 6 months, 67% at 1 year, 61% at 2 years and 58% at 3 years. Persistence data for both treatment duration scenarios could be found in Table 2.
The treatments cost included drug costs and costs for assessment. Drug costs were derived from official listings from February 2020. We also assigned the cost of one physician visit (€20.66) every 6 months of treatment (for persistent patients) and the cost of one bone density measurement (€43.36) at the start of treatment.
In line with previous economic analyses, the risks of gastrointestinal effects with alendronate and cellulitis with denosumab were considered. The assumptions relating to gastrointestinal effects were chosen to be similar to those used by the NICE appraisal. It was estimated that patients treated with ALN required 0.041 extra physician consultations during the first cycle (6 months) and 0.021 physician consultations during the following cycles on treatment, as well as a proton-pump inhibitor (PPI) for each visit. Despite studies suggested lower frequency of GI disorders with buffered soluble ALN, we conservatively assumed than buffered soluble ALN and generic ALN are associated with similar side effects in the base case. The rate of skin infections, including cellulitis, was reported more frequently with denosumab in the FREEDOM trial, i.e. 0.0031 annually, and was included in the analysis.
Persistence data
The Italian observational study included postmenopausal women from a standardized clinical database with BMD T-score < − 2.5, or between − 2 and − 2.5 and at least one vertebral fracture, starting buffered soluble ALN between July 2015 and June 2016. A historical cohort comprised of randomly selected and age-matched women on conventional ALN tablet was used as a control. Persistence at 6 and 12 months was estimated between the two groups.
Analyses
A total of 1,000,000 trials was run for each analysis. Total costs, disaggregated costs (i.e. treatment costs and fracture-related costs) and accumulated QALYs were estimated for each treatment. If buffered soluble ALN is associated with more QALYs and lower costs than an alternative treatment, buffered soluble ALN is considered dominant or cost-saving (when compared to no treatment). If buffered soluble ALN provides more QALYs and more costs, then we computed the incremental cost-effectiveness ratio (ICER) defined as the difference between buffered soluble ALN and the comparator treatment in terms of total costs (expressed in €2019) divided by the difference between them in terms of QALYs. If the ICER is above the cost-effectiveness threshold, then the cost is too high for the benefits and the intervention is not considered as cost-effective. In Italy, no specific threshold is actually used for defining cost-effectiveness. Borgström et al. [43] have suggested a threshold for QALY equal to two times the gross domestic product per capita for industrialized countries (±€70,000 in Italy). This assumption has been used for defining fracture risk thresholds in several countries [44].
Sensitivity analyses were then performed to assess the impact of model parameters on the results. One-way sensitivity analyses assessed the impact of single parameters on the results and were conducted on discount rates, fracture costs, fracture risks, fracture disutility, mortality and treatment costs. Sensitivity analyses were also conducted on varying by ± 25% the discontinuation rates of buffered soluble ALN and by reducing by half side effects associated with buffered soluble ALN. We also conducted another sensitivity analysis where treatment-specific efficacy data were derived from other sources. For ALN (used in our study for both generic ALN and buffered soluble ALN), data from the article of Black et al. (2000) were used [45]. Data of women with existing vertebral fracture were specifically used for this population, and data from women without vertebral fracture and femoral T-score < − 2.5 were used in the model for women with a T-score < − 3.0. The effects of zoledronic acid were derived from the HORIZON-FT trial [46], and the effects of denosumab were derived from a post hoc analysis of the FREEDOM trial in women with higher risk of fractures [47].
Finally, probabilistic sensitivity analyses were undertaken to examine the effect of the joint uncertainty surrounding the model variables. Nearly all parameters were modified simultaneously over plausible range of values, following guidelines and in line with previous studies (see Appendix 1 Table 2). For each probabilistic sensitivity analysis, the model was run 200 times based on runs of 50,000 patients per treatment arm. Results were presented in the form of cost-effectiveness acceptability curves that show the probability of being cost-effective as a function of the decision maker’s willingness to pay per QALY gained.
Results
Persistence data
A total of 360 postmenopausal women were included in the retrospective study; 144 were treated with buffered soluble ALN and 216 with conventional ALN tablet. The study revealed that a significantly higher number of women were persistent at 6 months and 12 months with buffered soluble ALN (91% and 81% respectively) compared to 75% and 69% of patients with conventional ALN tablet, respectively.
Base-case analysis
Table 3 presents the total and disaggregated healthcare costs, accumulated QALYs and the ICER (expressed in cost per QALY gained) of buffered soluble ALN compared with no treatment, generic ALN, denosumab and zoledronic acid in women aged 70 years. In the 1-year treatment scenario, in women with prevalent vertebral fractures, the incremental treatment cost (including drug cost adjusted by persistence and monitoring costs) between buffered soluble ALN and generic alendronate was €50, while the improved persistence of buffered soluble ALN leads to a saving of €39 resulting from more prevented fractures due to the improved persistence. The incremental total healthcare cost was thus estimated at +€11 (50–39) and buffered soluble ALN was associated with a QALY gain of 0.0028. The cost per QALY gained of buffered soluble ALN compared to generic alendronate was thus estimated at €4,028 (11/0.0028) per QALY gained. Compared to DMAB, buffered soluble ALN was associated with more QALYs and lower costs, being therefore dominant. Zoledronic acid is associated with more QALY than buffered soluble ALN; however, the cost per QALY gained of zoledronic acid (€121,514 per QALY gained) is higher than commonly accepted cost-effectiveness thresholds, meaning that zoledronic acid is not cost-effective compared to buffered soluble ALN. In women with BMD T-score ≤ − 3.0, buffered soluble ALN was dominant (more QALYs and lower costs) than generic alendronate and denosumab. In addition, buffered soluble ALN was cost-saving (more QALYs, lower total costs) compared to no treatment, and the cost per QALY gained of zoledronic compared to buffered soluble ALN was also higher than cost-effectiveness threshold, meaning that buffered soluble ALN is the most cost-effective option. In the 3-year treatment scenario, buffered soluble ALN was shown to be dominant compared to both denosumab and zoledronic acid in both populations. Buffered soluble ALN was also shown to be cost-saving and dominant compared to generic ALN in women with BMD T-score ≤ − 3.0.
In Table 4, the ICERs of buffered soluble ALN compared to all alternative treatments are presented for other ages ranging from 60 to 80 years. Appendix 2 Tables 1and 2 a-f provide the lifetime costs and QALYs for all these age-specific simulations. Compared to denosumab, buffered soluble ALN was always dominant (more QALYs, lower costs). The cost per QALY gained of buffered soluble ALN compared to generic ALN and no treatment falls always below €20,000 per QALY gained. In women aged 75 years and older with prevalent vertebral fractures and in women aged 65 years and older with T-score ≤ − 3.0, buffered soluble ALN was even shown to be dominant (more QALYs, lower costs) compared to generic alendronate and no treatment. In the 1-year treatment scenario, zoledronic acid was associated with more QALY than buffered soluble ALN but the cost per QALY gained of zoledronic acid compared to buffered soluble ALN was always higher than €70,000 per QALY gained, meaning that zoledronic acid is not cost-effective, while buffered soluble ALN was shown to be dominant compared to zoledronic acid in the 3-year treatment scenario.
Sensitivity analyses
Table 5 reports the results of the one-way sensitivity analyses in women aged 70 years with BMD T-score ≤ − 3.0 in the 1-year treatment scenario. In most analyses, buffered soluble ALN remained dominant (more QALYs, lower costs) compared to generic ALN and denosumab, and cost-saving compared to no treatment. The ICERs of buffered soluble ALN were shown to be in particular affected by fracture costs and discontinuation rates of buffered soluble ALN. In all the sensitivity analyses, buffered soluble ALN remained cost-effective compared to all treatments except when using other treatment-specific efficacy data where the ICER of zoledronic acid fall below the threshold of €70,000 per QALY gained.
The results of the probabilistic sensitivity analyses are provided in Fig. 1 where the cost-effectiveness acceptability curves show the probability that each intervention is cost-effective for different willingness to pay of decision makers per QALY gained. The curves suggest that buffered soluble ALN is the most cost-effective intervention for willingness to pay between €5,000 and €75,000 per QALY gained in the 1-year treatment xscenario. At a threshold of €45,000 per QALY gained, in women aged 70 years with BMD T-score ≤ − 3.0, buffered soluble ALN was cost-effective in 56% of the simulations compared to 6% for DMAB, 10% for generic alendronate and 26% for zoledronic acid. In the 3-year treatment scenario, buffered soluble ALN was the most cost-effective treatment for any willingness to pay.
Cost-effectiveness acceptability curve of buffered soluble ALN compared to individual treatments could be found in Appendix 2 Fig. 2 a, b, c, d for women with BMD T-score ≤ − 3.0 aged 70 years. Buffered soluble ALN was the most cost-effective intervention compared to all individual treatments. By example, at a threshold of €45,000 per QALY gained, in the 1-year treatment scenario, buffered soluble ALN was cost-effective in 100% of the simulations compared to no treatment, in 84% compared to generic alendronate, in 92% compared to denosumab and 71% compared to zoledronic acid.
Discussion
In the current research, we assessed the cost-effectiveness of buffered soluble ALN compared with relevant alternative treatments for postmenopausal osteoporotic women in Italy. Two treatment duration scenarios were assessed: a 1-year treatment scenario in line with available persistence data and a 3-year treatment scenario to better reflect clinical practices, extrapolating the 1-year persistence benefit of buffered soluble ALN up to 3 years. The results indicated that in both scenarios, buffered soluble ALN represents a cost-effective strategy compared with generic ALN, zoledronic acid, denosumab and no treatment for the treatment of postmenopausal women with osteoporosis in Italy aged 60 years and over. More specifically, buffered soluble ALN was shown to be dominant (more QALYs, lower costs) compared to denosumab. The cost per QALY gained of buffered soluble ALN compared to generic alendronate and no treatment always falls below €20,000 per QALY gained. In women aged 75 years and older with prevalent vertebral fractures and in women aged 65 years and older with T-score ≤ − 3.0, buffered soluble ALN was even shown to be dominant (more QALYs, lower costs) compared to generic alendronate and no treatment. In the 1-year treatment scenario, one yearly zoledronic acid was associated with full persistence and thus more QALY than buffered soluble ALN but the cost per QALY gained of zoledronic acid compared to buffered soluble ALN was higher than the cost-effectiveness threshold. In the 3-year treatment scenario, buffered soluble ALN was shown to be dominant compared to zoledronic acid.
To our knowledge, this study provides the first results about the cost-effectiveness of an effervescent alendronate for the treatment of postmenopausal women with osteoporosis. Buffered soluble ALN effervescent tablet was shown to be associated to a lower frequency of GI adverse reactions and greater medication persistence [9, 38]. Based on this improved persistence, our economic analysis suggests that the additional health benefits of buffered soluble ALN are worth for the additional drug cost compared to generic ALN [13]. Our analysis also suggested that buffered soluble ALN was associated with better outcomes and lower total costs than denosumab, resulting mainly from the absence of effects after discontinuation with denosumab. Recent evidence has indeed suggested an immediate bone loss after denosumab discontinuation and increased risk of vertebral fractures [34].
The results of the current study have to be interpreted within the context of some limitations. First, persistence data to buffered soluble ALN and generic alendronate were derived from one study with a total sample of 360 with similar characteristics than patients assessed in this economic study, and up to 1 year. Furthermore, the 1-year persistence benefit of buffered soluble ALN compared to oral ALN was extrapolated in the 3-year treatment scenario. Further studies with larger sample and longer follow-up would be needed to confirm the (long-term) persistence benefits of buffered soluble ALN. It is also important to acknowledge that persistence data and drug cost were derived from Binosto® buffered soluble ALN, and our finding could therefore not be applied to other formulation of ALN. Furthermore, it would be interesting to assess persistence for all medications in the same population. As patients on denosumab should continue denosumab therapy for up to 10 years or be switched to an alternative treatment (such as a bisphosphonate therapy) [48], long-term (sequential) treatment scenarios would also be interesting.
Second, our economic analysis was conducted in women with BMD T-score ≤ − 3.0 or with existing vertebral fractures in line with current reimbursement criteria for osteoporotic treatments in Italy. Further assessment of the cost-effectiveness of buffered soluble ALN in other populations (e.g. based on FRAX® score, or in patients with an imminent risk fracture) could be interesting. Other potential limitations are related to the model and data. The most important are availability of data. Although data used to construct the model were based on Italian literature whenever possible, some data were derived from other countries. In particular, no utility values were available in Italy and were therefore derived from the largest multinational study assessing the effects of fractures on quality of life (ICUROS). Conservative assumptions were further used for the cost of non-hip fractures. Support of an Italian expert and contact with the Italian National Institute of Health also helped to identify the best available data.
On the other hand, some assumptions were conservative for buffered soluble ALN treatment. First, similar side effects for buffered soluble ALN and generic alendronate were assumed, despite that buffered soluble ALN was associated with a lower frequency of GI adverse reactions [9]. A sensitivity analysis reducing by 50% side effects of buffered soluble ALN revealed however a very small effect of side effects on the ICER. Second, persistence data from the Italian observational database were derived for branded alendronate and used for the generic formulation in our economic study. Previous studies have suggested that persistence to generic formulations is even poorer than for branded formulations [49].
In conclusion, this study provides the first economic analysis of a buffered soluble ALN effervescent tablet, suggesting that buffered soluble ALN may represent a cost-effective strategy compared with relevant alternative treatments for the treatment of postmenopausal women with osteoporosis in Italy aged 60 years and over.
References
Borgstrom F, Karlsson L, Ortsater G, Norton N, Halbout P, Cooper C et al (2020) Fragility fractures in Europe: burden, management and opportunities. Arch Osteoporos 15(1):59
Kanis JA, Cooper C, Rizzoli R, Reginster JY, Scientific Advisory Board of the European Society for C, Economic Aspects of O et al (2019) Executive summary of European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Aging Clin Exp Res 31(1):15–17
Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T et al (2012) A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 73(5):691–705
Hiligsmann M, Cornelissen D, Vrijens B, Abrahamsen B, Al-Daghri N, Biver E et al (2019) Determinants, consequences and potential solutions to poor adherence to anti-osteoporosis treatment: results of an expert group meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the International Osteoporosis Foundation (IOF). Osteoporos Int 30(11):2155–2165
Hiligsmann M, Rabenda V, Gathon HJ, Ethgen O, Reginster JY (2010) Potential clinical and economic impact of nonadherence with osteoporosis medications. Calcif Tissue Int 86(3):202–210
Yeam CT, Chia S, Tan HCC, Kwan YH, Fong W, Seng JJB (2018) A systematic review of factors affecting medication adherence among patients with osteoporosis. Osteoporos Int 29(12):2623–2637
McClung MR, Miller PD, Brown JP, Zanchetta J, Bolognese MA, Benhamou CL et al (2012) Efficacy and safety of a novel delayed-release risedronate 35 mg once-a-week tablet. Osteoporos Int 23(1):267–276
Hodges LA, Connolly SM, Winter J, Schmidt T, Stevens HN, Hayward M et al (2012) Modulation of gastric pH by a buffered soluble effervescent formulation: a possible means of improving gastric tolerability of alendronate. Int J Pharm 432(1-2):57–62
Fardellone P, Boëzennec B, Cortet B (2019) Upper gastrointestinal safety with the buffered solution of alendronate 70 mg: 6 years of post-marketing experience. Osteoporos Int 30(2):S253–S773
Hiligsmann M, Evers SM, Ben Sedrine W, Kanis JA, Ramaekers B, Reginster JY, Silverman S, Wyers CE, Boonen A (2015) A systematic review of cost-effectiveness analyses of drugs for postmenopausal osteoporosis. Pharmacoeconomics 33(3):205–224
Hiligsmann M, Reginster JY, Tosteson ANA, Bukata SV, Saag KG, Gold DT, Halbout P, Jiwa F, Lewiecki EM, Pinto D, Adachi JD, al-Daghri N, Bruyère O, Chandran M, Cooper C, Harvey NC, Einhorn TA, Kanis JA, Kendler DL, Messina OD, Rizzoli R, Si L, Silverman S (2019) Recommendations for the conduct of economic evaluations in osteoporosis: outcomes of an experts’ consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the US branch of the International Osteoporosis Foundation. Osteoporos Int 30(1):45–57
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D et al (2013) Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Value Health 16(2):e1–e5
Hiligsmann M, Reginster JY (2019) Cost-effectiveness of gastro-resistant risedronate tablets for the treatment of postmenopausal women with osteoporosis in France. Osteoporos Int 30(3):649–658
Hiligsmann M, Williams SA, Fitzpatrick LA, Silverman SS, Weiss R, Reginster JY (2019) Cost-effectiveness of sequential treatment with abaloparatide vs. teriparatide for United States women at increased risk of fracture. Semin Arthritis Rheum 49(2):184–196
Hiligsmann M, Williams SA, Fitzpatrick LA, Silverman SS, Weiss R, Reginster JY (2020) Cost-effectiveness of sequential treatment with abaloparatide followed by alendronate vs. alendronate monotherapy in women at increased risk of fracture: A US payer perspective. Semin Arthritis Rheum 50(3):394–400
Hiligsmann M, Ethgen O, Bruyere O, Richy F, Gathon HJ, Reginster JY (2009) Development and validation of a Markov microsimulation model for the economic evaluation of treatments in osteoporosis. Value Health 12(5):687–696
Svedbom A, Hernlund E, Ivergard M, Compston J, Cooper C, Stenmark J et al (2013) Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos 8:137
Unim B et al (2020) Trends in hip and distal femoral fracture rates in Italy from 2007 to 2017. Bone
Piscitelli P, Chitano G, Johannson H, Brandi ML, Kanis JA, Black DM (2013) Updated fracture incidence rates for the Italian version of FRAX(R). Osteoporos Int 24(3):859–866
Piscitelli P, Tarantino U, Chitano G, Argentiero A, Neglia C, Agnello N, Saturnino L, Feola M, Celi M, Raho C, Distante A, Brandi ML (2011) Updated incidence rates of fragility fractures in Italy: extension study 2002-2008. Clin Cases Miner Bone Metab 8(3):54–61
Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P et al (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35(2):375–382
Kanis JA, Johnell O, Oden A, Jonsson B, De Laet C, Dawson A (2000) Risk of hip fracture according to the World Health Organization criteria for osteopenia and osteoporosis. Bone 27(5):585–590
Hiligsmann M, Reginster JY (2011) Cost effectiveness of denosumab compared with oral bisphosphonates in the treatment of post-menopausal osteoporotic women in Belgium. Pharmacoeconomics 29(10):895–911
Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B (2004) Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int 15(2):108–112
Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK (2003) The components of excess mortality after hip fracture. Bone. 32(5):468–473
Piscitelli P, Neglia C, Feola M, Rizzo E, Argentiero A, Ascolese M, et al (2020) Updated incidence and costs of hip fractures in elderly Italian population. Aging Clin Exp Res 32:2587–2593
Svedbom A, Hadji P, Hernlund E, Thoren R, McCloskey E, Stad R, Stollenwerk B (2019) Cost-effectiveness of pharmacological fracture prevention for osteoporosis as prescribed in clinical practice in France, Germany, Italy, Spain, and the United Kingdom. Osteoporos Int 30(9):1745–1754
Cosman F, Nieves JW, Dempster DW (2017) Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res 32(2):198–202
Svedbom A, Borgstom F, Hernlund E, Strom O, Alekna V, Bianchi ML et al (2018) Quality of life for up to 18 months after low-energy hip, vertebral, and distal forearm fractures-results from the ICUROS. Osteoporos Int 29:557–566
Hiligsmann M, Ethgen O, Richy F, Reginster JY (2008) Utility values associated with osteoporotic fracture: a systematic review of the literature. Calcif Tissue Int 82(4):288–292
NICE 2020. Bisphosphonates for treating osteoporosis. Technology appraisal guidance. Last update 8 July 2019. Access on March 2020 from https://www.nice.org.uk/guidance/ta464/resources/bisphosphonates-for-treating-osteoporosis-pdf-82604905556677
Strom O, Landfeldt E, Garellick G (2015) Residual effect after oral bisphosphonate treatment and healthy adherer effects--the Swedish Adherence Register Analysis (SARA). Osteoporos Int 26(1):315–325
Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361(8):756–765
Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guanabens N et al (2017) Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 105:11–17
Hiligsmann M, McGowan B, Bennett K, Barry M, Reginster JY (2012) The clinical and economic burden of poor adherence and persistence with osteoporosis medications in Ireland. Value Health 15(5):604–612
Hiligsmann M, Rabenda V, Bruyere O, Reginster JY (2010) The clinical and economic burden of non-adherence with oral bisphosphonates in osteoporotic patients. Health Policy 96(2):170–177
Hiligsmann M, Boonen A, Rabenda V, Reginster JY (2012) The importance of integrating medication adherence into pharmacoeconomic analyses: the example of osteoporosis. Expert Rev Pharmacoecon Outcomes Res 12(2):159–166
Giusti A, Bianchi G, Barone A, Black DM A novel effervescent formulation of oral weekly alendronate (70 mg) improves persistence compared to alendronate tablets in post-menopausal women with osteoporosis. Aging Clin Exp Res
Silverman SL, Siris E, Kendler DL, Belazi D, Brown JP, Gold DT, Lewiecki EM, Papaioannou A, Simonelli C, Ferreira I, Balasubramanian A, Dakin P, Ho P, Siddhanti S, Stolshek B, Recknor C (2015) Persistence at 12 months with denosumab in postmenopausal women with osteoporosis: interim results from a prospective observational study. Osteoporos Int 26(1):361–372
Tremblay E, Perreault S, Dorais M (2016) Persistence with denosumab and zoledronic acid among older women: a population-based cohort study. Arch Osteoporos 11(1):30
Koller G, Goetz V, Vandermeer B, Homik J, McAlister FA, Kendler D et al (2020) Persistence and adherence to parenteral osteoporosis therapies: a systematic review. Osteoporos Int 31(11):2093–2102
Fatoye F, Smith P, Gebrye T, Yeowell G (2019) Real-world persistence and adherence with oral bisphosphonates for osteoporosis: a systematic review. BMJ Open 9(4):e027049
Borgstrom F, Johnell O, Kanis JA, Jonsson B, Rehnberg C (2006) At what hip fracture risk is it cost-effective to treat? International intervention thresholds for the treatment of osteoporosis. Osteoporos Int 17(10):1459–1471
Lippuner K, Johansson H, Borgstrom F, Kanis JA, Rizzoli R (2012) Cost-effective intervention thresholds against osteoporotic fractures based on FRAX(R) in Switzerland. Osteoporos Int 23(11):2579–2589
Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S, Cummings SR, Fracture Intervention Trial (2000) Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab 85(11):4118–4124
Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356(18):1809–1822
Boonen S, Adachi JD, Man Z, Cummings SR, Lippuner K, Torring O et al (2011) Treatment with denosumab reduces the incidence of new vertebral and hip fractures in postmenopausal women at high risk. J Clin Endocrinol Metab 96(6):1727–1736
Kanis JA, Cooper C, Rizzoli R, Reginster JY, Scientific Advisory Board of the European Society for C, Economic Aspects of O et al (2019) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 30(1):3–44
Kanis JA, Reginster JY, Kaufman JM, Ringe JD, Adachi JD, Hiligsmann M, Rizzoli R, Cooper C (2012) A reappraisal of generic bisphosphonates in osteoporosis. Osteoporos Int 23(1):213–221
Acknowledgements
The authors are grateful to the Prince Mutaib Chair for Biomarkers of Chronic Disease, King Saud University, Riyadh, Saudi Arabia, for its support.
Funding
This work was funded by EffRx Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
MH has received research grant support (all through institution) from Amgen, Bayer, Radius Health, ViiV Healthcare and Interface Science & Research, and lecture fees from Teva. JYR has received consulting fees or paid advisory boards from IBSA-Genevrier, Mylan, Radius Health, Pierre Fabre, Teva; lecture fees when speaking at the invitation of sponsor: IBSA-Genevrier, Mylan, CNIEL, Dairy Research Council, Teva; grant support from industry (all through institution) from IBSA-Genevrier, Mylan, CNIEL, Radius Health. SM has received grants from Sanofi Pasteur, MSD, GSK, Pfizer, Takeda for taking part to advisory boards, expert meetings, for acting as speaker and (through institution) as organizer of meetings/congresses and as principal investigator of epidemiological studies.. The other authors have no conflict of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 58 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hiligsmann, M., Maggi, S., Veronese, N. et al. Cost-effectiveness of buffered soluble alendronate 70 mg effervescent tablet for the treatment of postmenopausal women with osteoporosis in Italy. Osteoporos Int 32, 595–606 (2021). https://doi.org/10.1007/s00198-020-05802-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-020-05802-5