Abstract
Purpose
Septic arthritis is a significant complication following arthroscopic surgery, with an estimated overall incidence of less than 1%. Despite the low incidence, an appropriate diagnostic and therapeutic pathway is required to avoid serious long-term consequences, eradicate the infection, and ensure good treatment outcomes. The aim of this current review article is to summarize evidence-based literature regarding diagnostic and therapeutic options of post-operative septic arthritis after arthroscopy.
Methods
Through a literature review, up-to-date treatment algorithms and therapies have been identified. Additionally, a supportive new algorithm is proposed for diagnosis and treatment of suspected septic arthritis following arthroscopic intervention.
Results
A major challenge in diagnostics is the differentiation of the post-operative status between a non-infected hyperinflammatory joint versus septic arthritis, due to clinical symptoms, (e.g., rubor, calor, or tumor) can appear identical. Therefore, joint puncture for microbiological evaluation, especially for fast leukocyte cell-count diagnostics, is advocated. A cell count of more than 20.000 leukocyte/µl with more than 70% of polymorphonuclear cells is the generally accepted threshold for septic arthritis.
Conclusion
The therapy is based on arthroscopic or open surgical debridement for synovectomy and irrigation of the joint, in combination with an adequate antibiotic therapy for 6–12 weeks. Removal of indwelling hardware, such as interference screws for ACL repair or anchors for rotator cuff repair, is recommended in chronic cases.
Level of evidence
IV.
Similar content being viewed by others
Introduction
In recent years, arthroscopic interventions have had a revolutionary impact on treating joint pathologies. Due to the technical improvements in arthroscopy, more and more pathologies are treated with a minimally invasive approach enabling the surgeon to have the best view during surgical intervention. As with every surgery, there is always the risk of infection, which has a major impact on the clinical outcome of every patient treated, through an arthroscopic technique. Even though it is considered to be a rare complication with an overall estimated incidence of less than 1%, the timely diagnosis and treatment of an infected joint is extremely important for successful management [7, 70]. However, an analysis of 15,167 patients after knee and shoulder arthroscopy showed that 37.1% of patients were readmitted within 30 days post-surgery due to an infection, underscoring the importance of post-surgical septic arthritis [82].
Epidemiology and pathophysiology
Shoulder
The infection can evolve via a hematogenous scattering or direct entry into the immune-privileged joint, which will be the focus of this article. Following shoulder arthroscopy with the use of anchors or suture material, germs can find excellent conditions for settlement.[22, 72]. Previous studies have identified significant risk factors for shoulder joint infections, which account for 88% of the patients examined in these studies (Table 1) [6, 13, 42].
Predominantly, shoulder infections are iatrogenic through peri- or intra-articular infiltration, as well as through surgical interventions [29]. There is an increased likelihood of shoulder joint infection in open procedures [24, 38] compared to purely arthroscopic procedures, that have an overall post-operative overall of around 1% [7, 84] and ranges from 0.16 to 2.10%, if it includes revision arthroscopic interventions [11, 54, 55, 58, 76, 84]. Almost all infections with a positive germ cultivation showed the presence of Cutibacterium acnes or Staphylococci. However, some other germs such as Pseudomonas aeruginosa, Mycobacterium tuberculosis, and Actinomyces have also been reported. There is an ongoing discussion about the use of pre-operative skin disinfectants and whether this has an influence on the post-operative infection rate. Therefore, Saltzmann et al. [61] investigated the use of povidone-iodine and showed an increased colonization in 31% of the cases following skin disinfection, 19% after iodophor-isopropyl alcohol disinfection and 7% after chlorhexidine-isopropyl alcohol disinfection. Additionally, a specific investigation regarding Cutibacterium acnes showed a persistence of colonization in 22.8% of the cases after pre-operative skin disinfection, with an increased colonization rate (42.6%) at the end of surgery [69]. In a time-related investigation, the odds ratio for a post-operative infection is 3.6 when surgery takes longer than 45 min with a more protective ratio for shorter interventions [11, 69]. To summarize the findings, the incidence of post-operative infection in shoulder arthroscopy is multifactorial and depends upon the type and time of surgery (primary or revision), and associated risk factors, and may also be influenced by the type of disinfectant,
Elbow joint infections
Despite the shift from open to arthroscopic procedures, the real incidence of infection at the beginning was unknown. Following a comprehensive analysis of 2704 Medicare patients treated with elbow arthroscopy, an incidence rate of 1.5% for deep infections has been reported [17]. There are only limited data regarding risk factors for elbow infection after arthroscopy, compared to knee and shoulder. However, it has been shown that alcohol use, inflammatory arthritis, hypercoagulability, age (> 65 years), diabetes mellitus, intra-articular corticosteroid, and obesity are significant risk factors [17, 81].
Wrist joint infections
Most of the data presented are referred to atraumatic septic arthritis with 2–5 infections per 100,000 in the general population and up to 38 per 100,000 individuals with rheumatoid arthritis [62]. The infection rate after arthroscopy can only be estimated from case series and is reported to be between 0 and 0.6% [8, 36, 67, 68, 83]. One study investigating the complications after wrist arthroscopy of 10,107 patients reported an incidence rate of 0.04% [45]. Furthermore, it has to be mentioned that the authors noted that infections were either not recorded or reported as infrequent [45].
Hip joint infections
Due to multifactorial etiologies of hip pain, intra-articular anesthetic or cortisone injection, as well as the injection of agents for MRI (gadolinium-based contrast agents) or CT have become essential tools in diagnosing hip pathologies. Wang et al. showed that there is a correlation between post-operative infection and pre-operative infiltration [78]. The closer the infiltration is performed prior to the surgical procedure, then the risk of infection increases after hip arthroscopy. The overall infection rate after hip arthroscopy was 1.1% (86/7620) with an elevated infection rate after injection of up to 2.8% with an injection less than 3 months before surgery (rate of control group was 1.1%) [78].
Summarizing the reported incidence for infection after hip arthroscopy in the literature, the rate is between 0 and 1.2%.[16, 20, 23, 26, 32, 34, 47, 53, 57, 75, 78, 79]. Regarding the risk factors for an infection, the reported factors are similar to the risk factors reported for shoulder arthroscopy (Table 2).
Knee joint infections
The overall reported infection rate is between 0.1 and 1.8%.[5, 10, 14, 18, 19, 21, 33, 37, 50, 66, 77]. This includes recent but also older literature. The real rate might be lower, as reported by meta-analysis of Cancienne et al.[19] and Yeranosian et al. [84, 85], who independently investigated the infection rate of over 1,000,000 patients after knee arthroscopy. They identified a post-arthroscopy infection risk between 0.15 and 0.46%, depending upon the cohort and the type of procedure. Additionally, demographic variables and comorbidities such as age (< 65), male gender, morbid obesity (BMI 40 +), tobacco use, diabetes mellitus, inflammatory arthritis, congestive heart failure, chronic kidney disease, hemodialysis, hypercoagulable disorder, and depression have been identified as independent risk factors for an infection after knee arthroscopy [19]. Besides the reported incidence and risk factors, there is a lot of information about the germs related to knee infection and Staphylococcus aureus is by far the most found bacteria [3, 5, 70]. However, some other germs such as coagulase-negative staphylococci (e.g., Staphylococcus epidermidis), MRSA, enterobacteria, streptococci, or fungal pathogens have been reported [3, 5, 70].
A significantly higher rate of post-surgical infections after arthroscopic ACL repair is described for professional athletes compared to amateur athletes [71]. However, papers by our group and others revealed no significant increase in infection rate after ACL reconstruction in professional athletes [12, 44].
Krutsch et al. described sports-related differences in infection rates after ACL injury and reconstruction [44]. Athletes practicing summer outdoor sports (e.g., football) had a significantly higher risk for infection after ACL reconstruction than winter sport athletes [44].
Ankle joint infections
Comprehensive registry analysis are missing, although the overall rate is reported to be between 0.13 and 1.8% [1, 9, 28, 29, 80]. It is even higher in patients who received an intraoperative intra-articular corticosteroid injection with an incidence rate of 3.9% [80].
Diagnosis
The difficulty in the diagnostic approach is to distinguish between a real post-operative septic arthritis and a post-operative hyperinflammation. The classical signs of infection such as joint swelling, reddening, overheating, pain, and limited range of motion (tumor, rubor, dolor, calor, and functio laesa) can be seen, whereas fever (possibly also chills) is more likely to be seen in septic arthritis. The diagnosis may not be obvious and mentioned signs of a joint infection can be masked [50]. Therefore, mild symptoms due to infection can be masked as signs of normal post-operative hyperinflammation [15, 40, 51]. According to Schollin-Borg et al.[64], in 60% of their cases after ACL reconstruction, the diagnosis of infection was missed at the patients’ first visit. Specifically, this is the case for patients with an indolent joint infection with non-aggressive or moderately aggressive germs, such as coagulase-negative Staphylococcus, and especially with Cutibacterium acnes after shoulder arthroscopy [50]. Additionally, gout arthritis should also be excluded, which can be done by the interpretation of blood infection parameters and examination results from joint fluid samples (joint fluid microscopy to confirm or exclude crystals).
After an inspection and palpation, the painful restricted range of motion can be the leading symptom during clinical examination [48]. Additionally, it is essential to distinguish between a joint irritation and a joint infection, especially after previous surgery (Table 3) [65].
Additional diagnostics
Even if there is little suspicion of an infected joint, blood tests should be initiated. Particular attention should be paid to the determination of the infection parameters such as leukocyte count, C-reactive protein (CRP), and the procalcitonin (PCT). Additionally, kidney and liver parameters should be determined, as they can be helpful to initiate and adapt a later antibiotic therapy. If there are signs of systemic infection, (e.g., fever), blood cultures should be taken at least in 2 pairs—2 aerobic and 2 anaerobic cultures from 2 different sites. However, the informative value of solely chemical blood tests, only shows a low specificity. The sensitivity can be increased by determining interleukin-6 in addition to CRP [65].
By ultrasound examination, a quick and easy-to-use procedure is available that allows for the detection of peri-articular fluid accumulation and joint effusions. However, it cannot distinguish between hyperinflammation and septic arthritis, as both show similar findings.
If infection is suspected, a conventional radiograph (2 plains) of the affected joint should also be carried out. This allows for assessment of any bony changes (e.g., osteolysis and osteophytes), as well as the evaluation of a physiological joint position and possible implants.
Extended diagnostic imaging with computed tomography (CT) or magnetic resonance imaging (MRI, with, i.v. injection of contrast medium) helps to further investigate the involvement of peri-articular soft-tissue structures and determination of an abscess. Positron emission tomography (PET)-CT and leukocyte scintigraphy are indicated to clarify unclear constellations of infection, especially in cases with unclear infection parameters, but are not used as a primary detection tool for joint infections.
The essential diagnostic tool for a suspected joint infection is the joint puncture. A sonographically assisted puncture is recommended and allows the controlled needle placement of the target area [2]. The procedure should be performed under sterile conditions (special room, disinfection, mouth protection, sterile gloves, and sterile drape). Afterward, the joint fluid sample should be assessed macroscopically (serous, clear, cloudy, and bloody) and then used for further determination of cell count, gram staining, microscopy, and extended microbiological diagnostics.
If an acute infection is suspected, cell count, macroscopic assessment, and microscopy after gram staining help to make a quick diagnosis (within hours) and support a quick decision-making process for further treatment (Fig. 1).
The interpretation of the joint fluid sample can be done according to Trampuz et al. [74] and Stutz et al. [73], who proposed the following criteria: the main distinctive feature between reactive and septic arthritis is the number of cells. If this is greater than 20,000/µl, there is a high probability of an infectious event (Table 3). However, there are some limitations to these criteria. The cell count must be interpreted with regard to the individual patient, i.e., a leukocyte count of 15,000/µl can already be considered critical if an intraarticular implant is present (anchor or suture material) and the cut-off value due to presence of joint replacements is even more strict (physiological < 2800 leukocytes/µl). Additionally, in patients with immunosuppression, the leucocyte count may not be elevated and therefore mask a joint infection.
This interpretation is also supported by the systematic review of Margaretten et al. [48] who showed that a progressively higher synovial white blood cell (WBC) count increased the likelihood of septic arthritis (Table 4). Additionally, they could show the importance of polymorphonuclear cells with an increased likelihood for septic arthritis when the percentage of polymorphonuclear cells is at least 90% (LR 3.4; 95% CI 2.8–4.2) [48]. If the polymorphonuclear cells are less than 90%, the likelihood decreased (LR 0.34; 95% CI 0.25–0.47) [48].
The negative results after cultivation, for assessment of joint fluid pathogens in the sample, do not necessarily exclude an infection. This also applies to the long-term cultivation (14 days and longer) [35].
Further microbiological diagnostics
In addition to the initially obtained joint fluid sample, revision arthroscopies should also collect at least 5 (tissue) samples for further microbiological investigation. The sensitivity for a germ detection is significantly increased with tissue samples compared to joint fluid only [87]. It should also be noted that bacterial detection is significantly less frequent with an ongoing antibiotic therapy. Therefore, if a joint infection is suspected, the main aim is to check for pathogens before starting an empirical i.v. antibiotic therapy. If the situation requires an implant removal during the revision surgery, it is recommended to prepare the implant(s) for an additional microbiological assessment using sonication. The sensitivity and specificity of sonication exceeds that of tissue biopsies (79% versus 61% for tissue biopsy) with a high specificity of 99% in total joint explants [59]
Additionally, positive microbiological results should also be interpreted with regards to a possible false-positive result and be discussed with the microbiologist and infectious disease specialist.
In any case, a long-term culture (at least 14 days) of the samples is recommended, as some pathogens can only be detected after this time period of cultivation. Specifically, Cutibacterium acnes is frequently detected in shoulder joint infections [49]. In state-of-the-art microbiological institutes, 16S ribosomal RNA PCR (polymerase chain reaction) can be used as a reliable (high sensitivity) and fast diagnostic tool that allows the detection of a broad range of pathogens with pathogen-specific PCR [46].
Classification of septic arthritis
Several classifications are available which evaluate the joint infection according to pathological, anatomical [25], clinical [73], or arthroscopic [30] aspects. The most frequently used classification with clinical relevance is the classification according to Gächter (Table 5, Fig. 2).
Therapeutic approach
If an infection is confirmed or suspected, an early arthroscopic joint irrigation and joint debridement should be performed (Fig. 2). The patient should be operated on within a few hours, if the patient has intervention-related fever and/or an increased cell-count analysis after joint puncture.
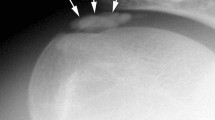
If a high-grade joint infection is already confirmed at the time of diagnosis (Gächter stage 4) by osteolysis using conventional radiography, an open procedure should be considered (Fig. 3) [27].
Algorithm for suspected joint infection after arthroscopy. In cases with indwelling implants, it is important to distinguish between an acute and chronic infection (see Table 4) in regard to implant preservation or removal (* in chronic cases mandatory, in acute cases helpful to identify implants and their position in case of surgery and subsequent removal if patient is not known to the presenting surgeon
At least five tissue samples should be obtained intraoperatively before starting a calculated antibiotic therapy. In addition, the histological examination is essential to support the diagnosis and to differentiate between septic arthritis between non-infectious joint pathologies (e.g., gout arthropathy) [39].
Surgical therapy
During revision, extensive lavage, debridement with synovectomy and hemostasis should be performed. Necrotic tissue or pannus tissue should be carefully removed.
In acute infections that are described in most arthroscopic case, an implant-retaining strategy with irrigation, debridement, and synovectomy followed by anti-biofilm antibiotic treatment should be targeted. In the case of chronic infections, complete hardware removal is necessary in most cases.
The surgical strategy aims to proceed according to the stage of the infection (Gächter I–IV). In the further post-operative course, a "second look" may be necessary. This mainly depends upon clinical signs and laboratory parameters. The intra-articular drain can give information about the joint fluid (clear or cloudy) and the infection blood parameters should drop after surgery (CRP, leucocytes, PCT) during antibiotic administration.
In the case of severe infection with residual infection parameters, a second surgery is required. It cannot be confirmed whether the repeated biopsy during the “second look” is clinically meaningful. Therefore, no recommendation can be made, as an ongoing antibiotic therapy will have a major impact on the microbiological results.
The intraoperative lavage should be carried out with a sufficient fluid volume (6 L of NaCl recommended). Antiseptics such as iodine-containing solutions, chlorhexidine, or hydrogen peroxide have good antimicrobial effects, but must not be used during surgical joint intervention due to their high chondrotoxicity that could lead to advanced chondrolysis [60, 63].
Drainage (with suction) is recommended to control the remaining intraarticular fluid and to have a direct visualization of the fluid itself, which may help to evaluate the post-operative clinical course [41]. The application of a suction–irrigation drainage or the application of a vacuum dressing is not recommended for intra-articular infections.
Antibiotic therapy
The administration of intra-articular antibiotics is not recommended, since the local effect level with systemic administration is above the minimum inhibitory concentration [52]. Additionally, there may also be an increased chondrotoxicity when administered locally.
Following adequate tissue and joint fluid collection, a calculated systemic antibiotic therapy must be started intravenously. In the absence of other risk factors, a second-generation cephalosporin is recommended for an antibiotic therapy of joint infections. However, newer strategies suggest the expansion of the calculated antibiotic therapy and the "hit hard and early" strategy. This will include the i.v. application of piperacillin/tazobactam (3 g) or amoxicillin/clavulanic acid (2.2 g) three times a day [31, 77]. Particularly in cases of acute infections with the intention to preserve implants, a biofilm effective antibiotic, such as rifampicin (dry wounds), in combination with the calculated antibiosis is recommended [86].
After receiving the antibiogram, the specific antibiotic therapy should be performed. The choice of antibiotic, as well as the method of application (i.v. vs. p.o.) and duration of the therapy, always depend upon accompanying factors. These can be the duration and severity of the infection, as well as accompanying diseases of the patient [31]. Special therapy regimes must be implemented when detecting multi-resistant bacteria and a special attention is required for rifampicin and ciprofloxacin resistant bacteria, due to their importance in treating biofilms. Therefore, an interdisciplinary cooperation between multiple faculties is recommended to find the best treatment for the patient.
Aftercare
During the duration of post-operative care, the passive mobilization of the joint is of high importance and joints should not be immobilized [43]. After removing the drainage and the recovery of the infection parameters, a more passive-assistive therapy can be started. With further control of the infections and improvement of joint conditions, active mobilization can be started. The further rehabilitation treatment is then based on the intraoperative findings and the reconstructive procedures during surgery.
Conclusions
In conclusion, septic arthritis is a significant complication after arthroscopic surgery. A major challenge in diagnostics is the differentiation of the post-operative status between a non-infected hyperinflammatory joint versus septic arthritis. Therefore, joint puncture for microbiological evaluation and particularly for fast leukocyte cell-count diagnostics is advocated. A cell count of more than 2.000 leukocyte/µl with more than 70% of polymorphonuclear cells is the generally accepted threshold for septic arthritis. The therapy is based on an arthroscopic or open surgical approach in combination with an adequate antibiotic therapy for 6–12 weeks.
References
Ahn JH, Park D, Park YT, Park J, Kim YC (2019) What should we be careful of ankle arthroscopy? J Orthop Surg (Hong Kong) 27:2309499019862502
Aly AR, Rajasekaran S, Ashworth N (2015) Ultrasound-guided shoulder girdle injections are more accurate and more effective than landmark-guided injections: a systematic review and meta-analysis. Br J Sports Med 49:1042–1049
Ascione T, Balato G, Mariconda M, Rosa D, Rizzo M, Pagliano P (2019) Post-arthroscopic septic arthritis of the knee. Analysis of the outcome after treatment in a case series and systematic literature review. Eur Rev Med Pharmacol Sci 23:76–85
Athwal GS, Sperling JW, Rispoli DM, Cofield RH (2007) Deep infection after rotator cuff repair. J Shoulder Elbow Surg 16:306–311
Balato G, Di Donato SL, Ascione T, D’Addona A, Smeraglia F, Di Vico G et al (2017) Knee septic arthritis after arthroscopy: incidence, risk factors, functional outcome, and infection eradication rate. Joints 5:107–113
Barzaga RA, Nowak PA, Cunha BA (1991) Escherichia coli septic arthritis of a shoulder in a diabetic patient. Heart Lung 20:692–693
Bauer T, Boisrenoult P, Jenny JY (2015) Post-arthroscopy septic arthritis: current data and practical recommendations. Orthop Traumatol Surg Res 101:S347-350
Beredjiklian PK, Bozentka DJ, Leung YL, Monaghan BA (2004) Complications of wrist arthroscopy. J Hand Surg Am 29:406–411
Blazquez Martin T, Iglesias Duran E, San Miguel Campos M (2016) Complications after ankle and hindfoot arthroscopy. Rev Esp Cir Ortop Traumatol 60:387–393
Boddapati V, Fu MC, Nwachukwu BU, Camp CL, Spiker AM, Williams RJ et al (2020) Procedure length is independently associated with overnight hospital stay and 30-day readmission following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 28:432–438
Boddapati V, Fu MC, Schairer WW, Ranawat AS, Dines DM, Taylor SA et al (2018) Increased shoulder arthroscopy time is associated with overnight hospital stay and surgical site infection. Arthroscopy 34:363–368
Bohu Y, Klouche S, Herman S, de Pamphilis O, Gerometta A, Lefevre N (2019) Professional athletes are not at a higher risk of infections after anterior cruciate ligament reconstruction: incidence of septic arthritis, additional costs, and clinical outcomes from the French prospective anterior cruciate ligament study (FAST) cohort. Am J Sports Med 47:104–111
Bonnaire F, Weber A (2014) S1-Leitlinie 012/010: Bakterielle Gelenkinfektionen, Association of the Scientific Medical Societies in Germany, registry number 012—010, https://www.awmf.org/leitlinien/detail/ll/012-010.html
Brophy RH, Wright RW, Huston LJ, Nwosu SK, Group MK, Spindler KP (2015) Factors associated with infection following anterior cruciate ligament reconstruction. J Bone Jt Surg Am 97:450–454
Burks RT, Friederichs MG, Fink B, Luker MG, West HS, Greis PE (2003) Treatment of post-operative anterior cruciate ligament infections with graft removal and early reimplantation. Am J Sports Med 31:414–418
Byrd JWT, Bardowski EA, Civils AN, Parker SE (2019) The safety of hip arthroscopy within 3 months of an intra-articular injection. J Bone Jt Surg Am 101:1467–1469
Camp CL, Cancienne JM, Degen RM, Dines JS, Altchek DW, Werner BC (2017) Factors that increase the risk of infection after elbow arthroscopy: analysis of patient demographics, medical comorbidities, and steroid injections in 2,704 medicare patients. Arthroscopy 33:1175–1179
Cancienne JM, Brockmeier SF, Carson EW, Werner BC (2018) Risk factors for infection after shoulder arthroscopy in a large medicare population. Am J Sports Med 46:809–814
Cancienne JM, Mahon HS, Dempsey IJ, Miller MD, Werner BC (2017) Patient-related risk factors for infection following knee arthroscopy: an analysis of over 700,000 patients from two large databases. Knee 24:594–600
Clarke MT, Arora A, Villar RN (2003) Hip arthroscopy: complications in 1054 cases. Clin Orthop Relat Res. 406:84–88
Clement RC, Haddix KP, Creighton RA, Spang JT, Tennant JN, Kamath GV (2016) Risk factors for infection after knee arthroscopy: analysis of 595,083 cases from 3 United States Databases. Arthroscopy 32:2556–2561
Connaughton A, Childs A, Dylewski S, Sabesan VJ (2014) Biofilm disrupting technology for orthopedic implants: what’s on the horizon? Front Med (Lausanne) 1:22
Cvetanovich GL, Chalmers PN, Levy DM, Mather RC 3rd, Harris JD, Bush-Joseph CA et al (2016) Hip arthroscopy surgical volume trends and 30-day post-operative complications. Arthroscopy 32:1286–1292
Day M, Westermann R, Duchman K, Gao Y, Pugely A, Bollier M et al (2018) Comparison of short-term complications after rotator cuff repair: open versus arthroscopic. Arthroscopy 34:1130–1136
Draijer F, Lorentzen T, Nissen R, Havemann D (1994) Die funktionelle Behandlung des operierten Kniegelenkempyems. Unfallchirurg 97:273–277
Du JY, Knapik DM, Trivedi NN, Sivasundaram L, Mather RC 3rd, Nho SJ et al (2019) Unplanned admissions following hip arthroscopy: incidence and risk factors. Arthroscopy 35:3271–3277
Enderle E, Frosch KH (2013) Stage-dependent arthroscopic treatment of knee joint infections. Oper Orthop Traumatol 25:225–235
Epstein DM, Black BS, Sherman SL (2015) Anterior ankle arthroscopy: indications, pitfalls, and complications. Foot Ankle Clin 20:41–57
Ferkel RD, Small HN, Gittins JE (2001) Complications in foot and ankle arthroscopy. Clin Orthop Relat Res. 391:89–104
Gächter A (1994) Gelenkinfekt - Arthroskopische Spülungsbehandlung - Hints und Tricks. Arthroskopie 7:98–101
Garrigues GE, Zmistowski B, Cooper AM, Green A, Group ICMS (2019) Proceedings from the 2018 International Consensus Meeting on Orthopedic Infections: management of periprosthetic shoulder infection. J Shoulder Elbow Surg 28:S67–S99
Griffin DR, Dickenson EJ, Wall PDH, Achana F, Donovan JL, Griffin J et al (2018) Hip arthroscopy versus best conservative care for the treatment of femoroacetabular impingement syndrome (UK FASHIoN): a multicentre randomised controlled trial. Lancet 391:2225–2235
Group M, Brophy RH, Wright RW, Huston LJ, Haas AK, Allen CR, et al. (2020) Rate of infection following revision anterior cruciate ligament reconstruction and associated patient- and surgeon-dependent risk factors: Retrospective results from MOON and MARS data collected from 2002 to 2011. J Orthop Res 39(2):274–280
Hartwell MJ, Morgan AM, Johnson DJ, Nicolay RW, Selley RS, Tjong VK et al (2020) Risk factors for 30-day readmission following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc 28:1290–1295
Hecker A, Jungwirth-Weinberger A, Bauer MR, Tondelli T, Uckay I, Wieser K (2020) The accuracy of joint aspiration for the diagnosis of shoulder infections. J Shoulder Elbow Surg 29:516–520
Hoel RJ, Mittelsteadt MJ, Samborski SA, Bohn DC (2018) Preoperative antibiotics in wrist arthroscopy. J Hand Surg Am 43(987–991):e981
Indelli PF, Dillingham M, Fanton G, Schurman DJ (2002) Septic arthritis in post-operative anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 398:182–188
Jensen AR, Cha PS, Devana SK, Ishmael C, Pauli Di, von Treuheim T, D’Oro A et al (2017) Evaluation of the trends, concomitant procedures, and complications with open and arthroscopic rotator cuff repairs in the medicare population. Orthop J Sports Med 5:2325967117731310
Jeon IH, Choi CH, Seo JS, Seo KJ, Ko SH, Park JY (2006) Arthroscopic management of septic arthritis of the shoulder joint. J Bone Jt Surg Am 88:1802–1806
Johnson MW (2000) Acute knee effusions: a systematic approach to diagnosis. Am Fam Physician 61:2391–2400
Jung HJ, Song JH, Kekatpure AL, Adikrishna A, Hong HP, Lee WJ et al (2016) The use of continuous negative pressure after open debridement for septic arthritis of the shoulder. Bone Jt J 98-B:660–665
Kirchhoff C, Braunstein V, Buhmann Kirchhoff S, Oedekoven T, Mutschler W, Biberthaler P (2009) Stage-dependant management of septic arthritis of the shoulder in adults. Int Orthop 33:1015–1024
Kirchhoff C, Braunstein V, Paul J, Imhoff AB, Hinterwimmer S (2009) Septic arthritis as a severe complication of elective arthroscopy:clinical management strategies. Patient Saf Surg 3:6
Krutsch W, Zellner J, Zeman F, Nerlich M, Koch M, Pfeifer C et al (2017) Sports-specific differences in postsurgical infections after arthroscopically assisted anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 25:3878–3883
Leclercq C, Mathoulin C, Members of E (2016) Complications of wrist arthroscopy: a multicenter study based on 10,107 arthroscopies. J Wrist Surg 5:320–326
Levy PY, Fenollar F (2012) The role of molecular diagnostics in implant-associated bone and joint infection. Clin Microbiol Infect 18:1168–1175
Malviya A, Raza A, Jameson S, James P, Reed MR, Partington PF (2015) Complications and survival analyses of hip arthroscopies performed in the national health service in England: a review of 6,395 cases. Arthroscopy 31:836–842
Margaretten ME, Kohlwes J, Moore D, Bent S (2007) Does this adult patient have septic arthritis? JAMA 297:1478–1488
Mook WR, Klement MR, Green CL, Hazen KC, Garrigues GE (2015) The incidence of propionibacterium acnes in open shoulder surgery: a controlled diagnostic study. J Bone Jt Surg Am 97:957–963
Mouzopoulos G, Fotopoulos VC, Tzurbakis M (2009) Septic knee arthritis following ACL reconstruction: a systematic review. Knee Surg Sports Traumatol Arthrosc 17:1033–1042
Musso AD, McCormack RG (2005) Infection after ACL reconstruction: what happens when cultures are negative? Clin J Sport Med 15:381–384
Nelson JD (1971) Antibiotic concentrations in septic joint effusions. N Engl J Med 284:349–353
Nwachukwu BU, McFeely ED, Nasreddine AY, Krcik JA, Frank J, Kocher MS (2011) Complications of hip arthroscopy in children and adolescents. J Pediatr Orthop 31:227–231
Parnes N, DeFranco M, Wells JH, Higgins LD, Warner JJ (2013) Complications after arthroscopic revision rotator cuff repair. Arthroscopy 29:1479–1486
Pauzenberger L, Grieb A, Hexel M, Laky B, Anderl W, Heuberer P (2017) Infections following arthroscopic rotator cuff repair: incidence, risk factors, and prophylaxis. Knee Surg Sports Traumatol Arthrosc 25:595–601
Pfeifer CG, Voss A, Alt V (2020) Komplikationsmanagement der infizierten Schulter. Arthroskopie 33:143–148
Rahl MD, LaPorte C, Steinl GK, O’Connor M, Lynch TS, Menge TJ (2020) Outcomes after arthroscopic hip labral reconstruction: a systematic review and meta-analysis. Am J Sports Med 48:1748–1755
Randelli P, Castagna A, Cabitza F, Cabitza P, Arrigoni P, Denti M (2010) Infectious and thromboembolic complications of arthroscopic shoulder surgery. J Shoulder Elbow Surg 19:97–101
Renz N, Cabric S, Janz V, Trampuz A (2015) Sonication in the diagnosis of periprosthetic infections: significance and practical implementation. Orthopade 44:942–945
Rohner E, Kolar P, Seeger JB, Arnholdt J, Thiele K, Perka C et al (2011) Toxicity of antiseptics against chondrocytes: what is best for the cartilage in septic joint surgery? Int Orthop 35:1719–1723
Saltzman MD, Nuber GW, Gryzlo SM, Marecek GS, Koh JL (2009) Efficacy of surgical preparation solutions in shoulder surgery. J Bone Jt Surg Am 91:1949–1953
Sammer DM, Shin AY (2011) Arthroscopic management of septic arthritis of the wrist. Hand Clin 27:331–334
Schneider MM, Preiss S, Harder LP, Salzmann GM (2015) Destructive chondrolysis following intraarticular application of lavasorb (polihexanid) for treatment of knee empyema. MMW Fortschr Med 157:47–49
Schollin-Borg M, Michaelsson K, Rahme H (2003) Presentation, outcome, and cause of septic arthritis after anterior cruciate ligament reconstruction: a case control study. Arthroscopy 19:941–947
Schumann K, Buchmann S, Paul P, Imhoff A (2013) Infekt nach Arthroskopie. Arthroskopie 26:259–266
Schuster P, Schulz M, Immendoerfer M, Mayer P, Schlumberger M, Richter J (2015) Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: evaluation of an arthroscopic graft-retaining treatment protocol. Am J Sports Med 43:3005–3012
Selles CA, d’Ailly PN, Schep NWL (2020) Patient-reported outcomes following arthroscopic triangular fibrocartilage complex repair. J Wrist Surg 9:58–62
Selles CA, Mulders MAM, Colaris JW, van Heijl M, Cleffken BI, Schep NWL (2020) Arthroscopic debridement does not enhance surgical treatment of intra-articular distal radius fractures: a randomized controlled trial. J Hand Surg Eur 45:327–332
Sethi PM, Sabetta JR, Stuek SJ, Horine SV, Vadasdi KB, Greene RT et al (2015) Presence of Propionibacterium acnes in primary shoulder arthroscopy: results of aspiration and tissue cultures. J Shoulder Elbow Surg 24:796–803
Sircana G, Passiatore M, Capasso L, Saccomanno MF, Maccauro G (2019) Infections in arthroscopy. Eur Rev Med Pharmacol Sci 23:279–287
Sonnery-Cottet B, Archbold P, Zayni R, Bortolletto J, Thaunat M, Prost T et al (2011) Prevalence of septic arthritis after anterior cruciate ligament reconstruction among professional athletes. Am J Sports Med 39:2371–2376
Stewart PS, Bjarnsholt T (2020) Risk factors for chronic biofilm-related infection associated with implanted medical devices. Clin Microbiol Infect 26:1034–1038
Stutz G (2005) Diagnostik und arthroskopische Therapie von Gelenkinfekten. SFA Arthroskopie Aktuell Nr 18:1–18
Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R (2004) Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med 117:556–562
Truntzer JN, Hoppe DJ, Shapiro LM, Abrams GD, Safran M (2017) Complication rates for hip arthroscopy are underestimated: a population-based study. Arthroscopy 33:1194–1201
Vopat BG, Lee BJ, DeStefano S, Waryasz GR, Kane PM, Gallacher SE et al (2016) Risk factors for infection after rotator cuff repair. Arthroscopy 32:428–434
Wang C, Lee YH, Siebold R (2014) Recommendations for the management of septic arthritis after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 22:2136–2144
Wang D, Camp CL, Ranawat AS, Coleman SH, Kelly BT, Werner BC (2017) The timing of hip arthroscopy after intra-articular hip injection affects post-operative infection risk. Arthroscopy 33(1988–1994):e1981
Weber AE, Harris JD, Nho SJ (2015) Complications in hip arthroscopy: a systematic review and strategies for prevention. Sports Med Arthrosc Rev 23:187–193
Werner BC, Cancienne JM, Burrus MT, Park JS, Perumal V, Cooper MT (2016) Risk of infection after intra-articular steroid injection at the time of ankle arthroscopy in a medicare population. Arthroscopy 32:350–354
Werner BC, Fashandi AH, Chhabra AB, Deal DN (2016) Effect of obesity on complication rate after elbow arthroscopy in a medicare population. Arthroscopy 32:453–457
Westermann RW, Pugely AJ, Ries Z, Amendola A, Martin CT, Gao Y et al (2015) Causes and predictors of 30-day readmission after shoulder and knee arthroscopy: an analysis of 15,167 cases. Arthroscopy 31(1035–1040):e1031
Wu M, Miller PE, Waters PM, Bae DS (2020) Early results of surgical treatment of triangular fibrocartilage complex tears in children and adolescents. J Hand Surg Am 45:449 e441-449 e449
Yeranosian MG, Arshi A, Terrell RD, Wang JC, McAllister DR, Petrigliano FA (2014) Incidence of acute post-operative infections requiring reoperation after arthroscopic shoulder surgery. Am J Sports Med 42:437–441
Yeranosian MG, Petrigliano FA, Terrell RD, Wang JC, McAllister DR (2013) Incidence of post-operative infections requiring reoperation after arthroscopic knee surgery. Arthroscopy 29:1355–1361
Zimmerli W, Sendi P (2017) Orthopaedic biofilm infections. APMIS 125:353–364
Zimmerli W, Trampuz A, Ochsner PE (2004) Prosthetic-joint infections. N Engl J Med 351:1645–1654
Acknowledgements
We would like to thank Dr. Girish Pattappa, being a native English speaker, for proofreading the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Voss, A., Pfeifer, C.G., Kerschbaum, M. et al. Post-operative septic arthritis after arthroscopy: modern diagnostic and therapeutic concepts. Knee Surg Sports Traumatol Arthrosc 29, 3149–3158 (2021). https://doi.org/10.1007/s00167-021-06525-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-021-06525-8