Abstract
Purpose
It was reported that not only ACL but also the synovium may be the major regulator of matrix metalloproteinases (MMPs) in synovial fluids after ACL injury. In order to further confirm whether synovium is capable of regulating the microenvironment in the process of ACL injury, the complicated microenvironment of joint cavity after ACL injury was mimicked and the combined effects of mechanical injury and inflammatory factor [tumour necrosis factor-α (TNF-α)] on expressions of lysyl oxidases (LOXs) and MMPs in synovial fibroblasts derived from normal human synovium were studied.
Methods
Human normal knee joint synovial fibroblasts were stimulated for 1–6 h with mechanical stretch and inflammatory factor (TNF-α). Total RNA was harvested, reverse transcribed and assessed by real-time polymerase chain reaction for the expression of LOXs and MMP-1, 2, 3 messenger RNAs. MMP-2 activity was assayed from the collected culture media samples using zymography.
Results
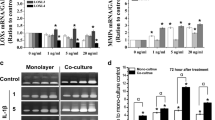
Compared to control group, our results showed that 6 % physiological stretch increased MMP-2 and LOXs (except LOXL-3), decreased MMP-1 and MMP-3; injurious stretch (12 %) decreased LOXs (except LOXL-2)and increased MMP-1, 2 and 3; the combination of injurious stretch and TNF-α decreased LOXs and increased MMP-1, 2 and 3 in synovial fibroblasts in a synergistical manner.
Conclusion
This study demonstrated that combination of mechanical injury and inflammatory factors up-regulated the expressions of MMPs and down-regulated the expressions of LOXs in synovial fibroblasts, eventually alter the balance of tissue healing. Thus, synovium may be involved in regulating the microenvironment of joint cavity. Based on the mechanism, early interventions to inhibit the production of MMPs or promote the production of LOXs in the synovial fibroblasts should be performed to facilitate the healing of tissue.







Similar content being viewed by others
References
Anitua E, Sanchez M, De la Fuente M et al (2012) Plasma rich in growth factors (PRGF-Endoret) stimulates tendon and synovial fibroblasts migration and improves the biological properties of hyaluronic acid. Knee Surg Sports Traumatol Arthrosc 20:1657–1665
Asundi KR, Rempel DM (2008) Cyclic loading inhibits expression of MMP-3 but not MMP-1 in an in vitro rabbit flexor tendon model. Clin Biomech 23:117–121
Bray RC, Leonard CA, Salo PT (2002) Vascular physiology and long-term healing of partial ligament tears. J Orthop Res 20:984–989
Breshears LA, Cook JL, Stoker AM et al (2010) The effect of uniaxial cyclic tensile load on gene expression in canine cranial cruciate ligamentocytes. Vet Surg 39:433–443
Catrina AI, Lampa J, Ernestam S et al (2002) TNF-α as a promising therapeutic target in chronic asthma: a lesson from rheumatoid arthritis. Rheumatology 41:484–489
Chamberlain CS, Brounts SH, Sterken DG et al (2011) Gene profiling of the rat medial collateral ligament during early healing using microarray analysis. J Appl Physiol 111:552–565
Chithra P, Sajithlal GB, Chandrakasan G (1998) Influence of Aloe vera on collagen characteristics in healing dermal wounds in rats. Mol Cell Biochem 181:71–76
DesRosiers EA, Yahia L, Rivard CH (1996) Proliferative and matrix synthesis response of canine anterior cruciate ligament fibroblasts submitted to combined growth factors. J Orthop Res 14:200–208
Di Sabatino A, Pender SL, Jackson CL et al (2007) Functional modulation of Crohn’s disease myofibroblasts by anti-tumor necrosis factor antibodies. Gastroenterology 133:137–149
Fisher JF, Mobashery S (2006) Recent advances in MMP inhibitor design. Cancer Metastasis Rev 25:115–136
Fleming BC, Beynnon BD (2004) In vivo measurement of ligament/tendon strains and forces: a review. Ann Biomed Eng 32:318–328
Fu FH, Bennett CH, Lattermann C et al (2000) Current trends in anterior cruciate ligament reconstruction. Am J Sports Med 28:124–130
Greenwel P, Tanaka S, Penkov D et al (2000) Tumor necrosis factor alpha inhibits type I collagen synthesis through repressive CCAAT/enhancer-binding proteins. Mol Cell Biol 20:912–918
Hou WS, Li Z, Gordon RE et al (2001) Cathepsin K is a critical protease in synovial fibroblast-mediated collagen degradation. Am J Pathol 159:2167–2177
Hsieh AH, Sah RL, Sung KL (2002) Biomechanical regulation of type I collagen gene expression in ACLs in organ culture. J Orthop Res 20:325–331
Hsieh AH, Tsai CMH, Ma QJ et al (2000) Time-dependent increase in type-III collagen gene expression in medial collateral ligament fibroblasts under cyclic strains. J Bone Joint Surg 18:220–227
Irie K, Uchiyama E, Iwaso H (2003) Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee 10:93–96
Ishiguro N, Shimizu T, Ito T et al (2000) The expression of matrix metalloproteinases and inhibitors in acute rupture of the anterior cruciate ligament. Mod Rheumatol 10:95–102
Kagan HM, Li W (2003) Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem 88:660–672
Kerrigan JJ, Mansell JP, Sandy JR (2000) Matrix turnover. J Orthod 27:227–233
Lee AA, Delhaas T, McCulloch AD et al (1999) Differential responses of adult rat cardiac fibroblasts to in vitro biaxial strain patterns. J Mol Cell Cardiol 31:1833–1843
Lee AA, Delhaas T, Waldman LK et al (1996) An equibiaxial strain system for cultured cells. Am J Physiol 271:C1400–C1408
Lohmander LS, Englund PM, Dahl LL, Dahl LL, Roos EM (2007) The long-term consequence of anterior cruciate ligament and meniscus injuries. Am J Sports Med 35:1756–1769
Muir P, Danova NA, Argyle DJ et al (2005) Collagenolytic protease expression in cranial cruciate ligament and stifle synovial fluid in dogs with cranial cruciate ligament rupture. Vet Surg 34:482–490
Murakami H, Shinomiya N, Kikuchi T et al (2006) Upregulated expression of inducible nitric oxide synthase plays a key role in early apoptosis after anterior cruciate ligament injury. J Orthop Res 24:1521–1534
Nagineni CN, Amiel D, Green MH et al (1992) Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res 10:465–475
Pap T, Müller-Ladner U, Gay RE et al (2000) Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res 2:361–367
Patwari P, Cook MN, DiMicco MA et al (2003) Proteoglycan degradation after injurious compression of bovine and human articular cartilage in vitro: interaction with exogenous cytokines. Arthritis Rheum 48:1292–1301
Poole AR (1997) Cartilage in health and disease. In: Koopman WJ (ed) Arthritis and allied conditions, 13th edn. Williams and Wilkins, Baltimore
Powers JC, Asgian JL, Ekici OD et al (2002) Irreversible Inhibitors of Serine, Cysteine, and Threonine Proteases. Chem Rev 102:4639–4750
Russo C, Polosa R (2005) Anti-tumor necrosis factor (TNF)-α therapy (etanercept) down-regulates serum matrix metalloproteinase (MMP)-3 and MMP-1 in rheumatoid arthritis. Clin Sci 109:135–142
Spindler KP, Clark SW, Nanney LB et al (1996) Expression of collagen and matrix metalloproteinases in ruptured human anterior cruciate ligament: an in situ hybridization study. J Orthop Res 14:857–861
Sung KL, Yang L, Whittemore DE et al (1996) The differential adhesion forces of anterior cruciate ligament and medial collateral ligament fibroblasts: effects of tropomodulin, talin, vinculin, and alpha-actinin. Proc Natl Acad Sci USA 93:9182–9187
Sun HB, Yokota H (2001) Messenger-RNA expression of matrix metalloproteinases, tissue inhibitors of metalloproteinases, and transcription factors in rheumatic synovial cells under mechanical stimuli. Bone 28:303–309
Tandara AA, Mustoe TA (2004) Oxygen in wound healing—more than a nutrient. World J Surg 28:294–300
Tang Z, Yang L, Wang Y et al (2009) Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res 27:243–248
Tchetverikov I, Lohmander LS, Verzijl N et al (2005) MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann Rheum Dis 64:694–698
Vater CA, Harris ED Jr, Siegel RC (1979) Native cross-links in collagen fibrils induce resistance to human synovial collagenase. Biochem J 181:639–645
Wang P, Yang L, You X et al (2009) Mechanical stretch regulates the expression of matrix metalloproteinases in rheumatoid arthritis fibroblast-like synoviocytes. Connect Tissue Res 50:98–109
Wang Y, Tang Z, Xue R et al (2011) Combined effects of TNF-α, IL-1β, and HIF-1α on MMP-2 production in ACL fibroblasts under mechanical stretch: an in vitro study. J Orthop Res 29:1008–1014
Wang Y, Yang L, Zhang J et al (2009) Differential MMP-2 activity induced by mechanical compression and inflammatory factors in human synoviocytes. Mol Cell Biomech 7:105–114
Wiig ME, Amiel D, Ivarsson M et al (1991) Type I procollagen gene expression in normal and early healing of the medial collateral and anterior cruciate ligaments in rabbits: an in situ hybridization study. J Orthop Res 9:374–382
Witkowski J, Yang L, Wood DJ et al (1997) Migration and healing of ligament cells under inflammatory conditions. J Orthop Res 15:269–277
Xie J, Jiang J, Zhang Y et al (2012) Up-regulation expressions of lysyl oxidase family in ACL and MCL fibroblasts induced by TGF-β1. Int Orthop 36:207–213
Xie J, Wang C, Yin L et al (2012) IL-1β influences on lysyl oxidases and matrix metalloproteinases profile of injured anterior cruciate ligament and medial collateral ligament fibroblasts. Int Orthop. PMID: 22588690
Yan C, Boyd DD (2007) Regulation of matrix metalloproteinase gene expression. J Cell Physiol 211:19–26
Yokota H, Goldring MB, Sun HB (2003) CITED2-mediated regulation of MMP-1 and MMP-13 in human chondrocytes under flow shear. J Biol Chem 278:47275
Zhou D, Lee HS, Villarreal F et al (2005) Differential MMP-2 activity of ligament cells under mechanical stretch injury: an in vitro study on human ACL and MCL fibroblasts. J Orthop Res 23:949–957
Acknowledgments
This study was supported by the Innovation and Attracting Talents Program for College and University (“111” Project) (B06023), NSF Projects (10672195, 30870607), CSTC2008BB5192, Sharing fund of Chongqing university’s large-scale equipment (2009063038) and by NIH AR45635 (USA).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Y., Huang, W., Jiang, J. et al. Influence of TNF-α and biomechanical stress on matrix metalloproteinases and lysyl oxidases expressions in human knee synovial fibroblasts. Knee Surg Sports Traumatol Arthrosc 22, 1997–2006 (2014). https://doi.org/10.1007/s00167-013-2425-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-013-2425-z