Abstract
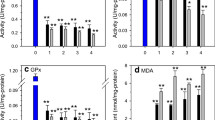
This study investigated the effect of persistent heavy metal exposure on the activity of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) of the freshwater snail, Lymnaea natalensis. Malondialdehyde (MDA) levels were also measured as an index of lipid peroxidation. The snails were exposed to cadmium, copper, lead and mercury for a total of 28 days at 0.1 mg/L, 0.1 mg/L, 0.2 mg/L and 0.1 mg/L respectively. Samples were collected at 1, 7, 14, 21 and 28 days intervals. Analysis of SOD showed significant initial increases in enzyme activity following exposure to copper, lead and mercury, while cadmium exposures caused increases from Day 14 onwards. Copper, cadmium and lead caused significant initial increases in CAT activity and mercury caused an increase only on Day 28. Copper caused a significant increase in GPx activity on Day 28 while MDA levels diminished significantly at Days 7–28. Similarly, cadmium caused significant increases of GPx activity on Day 28 whereas MDA levels were significantly reduced. Lead also caused a significant increase in GPx activity on Days 14–28 whilst no significant changes occurred in MDA levels. Mercury exposures caused significant increases in GPx activity on Days 7 and 21, whilst MDA levels were significantly reduced on Days 7 and 14. These findings suggest that persistent exposure of snails to heavy metals induces the antioxidant defence system, and decreases lipid peroxidation.




Similar content being viewed by others
References
Almar M, Otero L, Santos C, Galego JG (1998) Liver glutathione content and glutathione dependent enzymes of two species of freshwater fish as bioindicators of chemical pollution. J Environ Sci Health B33:769–783
Basopo N, Ngabaza T (2015) Toxicological effects of chlorpyrifos and lead on the aquatic snail Helisoma dyuri. Adv Biol Chem 5:225–233
Bhagat J, Ingole BS, Singh N (2016) Glutathione S-transferase, catalase, superoxide dismutase, glutathione peroxidase, and lipid peroxidation as biomarkers of oxidative stress in snails: a review. Invertebrate Surviv J 13:336–349
Chelomin VP, Belcheva NN (1992) The effect of heavy metals on processes of lipid peroxidation in microsomal membranes from the hepatopancreas of the bivalve mollusc Mizuhopecten yessoensis. Comp Biochem Physiol C 103:419–422
Clairborne A (1989) Catalase activity. In: Greenwald AR (ed) Handbook of methods of oxygen radical research. CRC Press, Florida, p 283
de Almeida EA, Miyamoto S, Bainy ACD, De Medeiros MHG, Di Mascio P (2004) Protective effect of phospholipid hydroperoxide glutathione peroxidase (PHGPx) against lipid peroxidation in mussels perna perna exposed to different metals. Mar Pollut Bull 49:386–392
De Voogt P (2016) Reviews of environmental contamination and toxicology, vol 237. Springer International Publishing, Switzerland
Draper HH, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol 186:421–431
Ercal N, Gurer-Orhan H, Aykin-Burns N (2001) Toxic metals and oxidative stress part I: mechanisms involved in metal-induced oxidative damage. Curr Top Med Chem 1:529–539
Fergusson JE (1990) The heavy elements: chemistry, environmental impact, and health effects. Pergamon Press, Oxford
Flohé L, Günzler WA (1984) Assays of glutathione peroxidase. Methods Enzymol 105:114–120
Goldstein RS, Hewitt WR, Hook JB (2013) Toxic interactions. Elsevier, Amsterdam
Gromadzińska J, Wasowicz W, Sktodowska M, Stróżyński H (1988) Glutathione peroxidase activity, lipid peroxides and selenium status in blood in patients with Down’s Syndrome. Clin Chem Lab Med 26:255–258
Han XY, Xu ZR, Wang YZ, Huang QC (2006) Effect of cadmium on lipid peroxidation and activities of antioxidant enzymes in growing pigs. Biol Trace Elem Res 110:251–263
Ho E, Karimi GK, Liu CC, Bhindi R, Figtree GA (2013) Biological markers of oxidative stress: applications to cardiovascular research and practice. Redox Biol 1:483–491
Jakimska A, Konieczka P, Skóra K, Namieśnik J (2011) Bioaccumulation of metals in tissues of marine animals, part I: the role and impact of heavy metals on organisms. Pol J of Environ Stud 20:1117–1125
Lei Y, Zhang W, Xu W, Zhang Y, Zhou H, Mai K (2015) Effects of waterborne Cu and Cd on anti-oxidative response, lipid peroxidation and heavy metals accumulation in abalone Haliotis discus hannai Ino. J Ocean Univ China 14:511–521
Li Z, Zlabek V, Velisek J, Grabic R, Machova J, Kolarova J, Li P, Randák T (2011) Antioxidant responses and plasma biochemical characteristics in the freshwater rainbow trout, Oncorhynchus mykiss, after acute exposure to the fungicide propiconazole. Czech J Anim Sci 56:61–69
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Manduzio H, Rocher B, Durand F, Galap C, Leboulenger F (2005) The point about oxidative stress in molluscs. Inf Syst 2:91–104
Mestry UD, Bhosale TS (2018) Mercury toxicity on lipid peroxidation in gill and hepatopancreas of freshwater bivalve Lamellidens corrianus. Bio Disc 9:100–103
Monteiro DA, Rantin FT, Kalinin AL (2009) Inorganic mercury exposure: toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxã, Brycon amazonicus (Spix and Agassiz 1829). Ecotoxicology 19:105
Naik YS, Hasler JA (2002) An economical method for the maintenance of schistosome life-cycles using outdoor facilities. Discov Innov 14:221–225
Packer L, Fuchs J (1992) Vitamin E in health and disease: biochemistry and clinical applications. CRC Press, Florida
Pandey S, Parvez S, Sayeed I, Haque R, Bin-Hafeez B, Raisuddin S (2003) Biomarkers of oxidative stress: a comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci Total Environ 309:105–115
Pruski AM, Dixon DR (2002) Effects of cadmium on nuclear integrity and DNA repair efficiency in the gill cells of Mytilus edulis L. Aquat Toxicol 57:127–137
Rajeswari RT, Sailaja N (2014) Impact of heavy metals on environmental pollution. J Chem Pharm Sci 3:175–181
Repetto M, Semprine J, Boveris A (2012) Lipid peroxidation: chemical mechanism, biological implications and analytical determination. In: Catala A (ed) Lipid peroxidation. IntechOpen, London, pp 3–30
Rodrigues-Ariza A, Abril N, Navas JJ, Dorado G, Lopez-Barea J, Pueyo C (1992) Metal mutagenicity and biochemical studies on bivalve molluscs from Spanish coasts. Environ Mol Mutagen 19:112–124
Roméo M, Gnassia-Barelli M (1997) Effect of heavy hetals on lipid peroxidation in the Mediterranean Clam Ruditapes decussatus. Comp Biochem Physiol C 118:33–37
Sevcikova M, Modra H, Slaninova A, Svobodova Z (2011) Metals as a cause of oxidative stress in fish: a review. Vet Med 56:537–546
Shireen SA, Nasr EN, Khaled AK, Hossam ES (2019) Hemotoxic and genotoxic effects of lead acetate and chlorpyrifose on freshwater cat fish (Calarias gariepinus). Slov Vet Res 2019:681–691
Siwela AH, Nyathi CB, Naik YS (2009) Metal accumulation and antioxidant enzyme activity in C. gariepinus, catfish and O. mossambicus, tilapia collected from lower mguza and wright dams. Zimbabwe Bull Environ Contam Toxicol 83:648–651
Siwela AH, Nyathi CB, Naik YS (2010) A comparison of metal levels and antioxidant enzymes in freshwater snails, Lymnaea natalensis, exposed to sediment and water collected from Wright Dam and Lower Mguza Dam, Bulawayo, Zimbabwe. Ecotoxicol Environ Saf 73:1728–1732
Stern BR (2010) Essentiality and toxicity in copper health risk assessment: overview, update and regulatory considerations. J Toxicol Environ Health, Part A 73:114–127
Stohs S, Bagchi D, Hassoun E, Bagchi M (2000) Oxidative mechanisms in the toxicity of chromium and cadmium ions. J Environ Pathol Toxicol Onco 19:201–213
Sun Y, Larry WO, Ying L (1988) A simple method for clinical assay of superoxide dismutase. Clin Chem 34:497–500
Thomas J, Maiorino M, Ursini F, Girotti A (1990) Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation. In situ reduction of phospholipid and cholesterol hydroperoxides. J Biol Chem 265:454–461
Tkachenko H, Kurhaluk N, Grudniewska J, Andriichuk A (2014) Tissue-specific responses of oxidative stress biomarkers and antioxidant defenses in rainbow trout Oncorhynchus mykiss during a vaccination against furunculosis. Fish Physiol Biochem 40:1289–1300
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64:178–189
Acknowledgements
This research was supported by the International Programme in Chemical Sciences (IPICS), Uppsala University, Sweden and the Research and Innovation Board, National University of Science and Technology, Zimbabwe.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mnkandla, S.M., Basopo, N. & Siwela, A.H. The Effect of Persistent Heavy Metal Exposure on Some Antioxidant Enzyme Activities and Lipid Peroxidation of the Freshwater snail, Lymnaea natalensis. Bull Environ Contam Toxicol 103, 551–558 (2019). https://doi.org/10.1007/s00128-019-02693-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-019-02693-z