Abstract
Aims/hypothesis
Physical activity may increase a person’s inhalation of air pollutants and exacerbate the adverse health effects. This study aimed to investigate the combined associations of chronic exposure to particulate matter with an aerodynamic diameter less than 2.5 μm (PM2.5) and habitual physical activity with the incidence of type 2 diabetes in Taiwan.
Methods
We selected 156,314 non-diabetic adults (≥18 years old) who joined an ongoing longitudinal cohort between 2001 and 2016. Incident type 2 diabetes was identified at the follow-up medical examinations. Two-year mean PM2.5 exposure was estimated at each participant’s address using a satellite-based spatiotemporal model. Information on physical activity and a wide range of covariates was collected using a standard self-administered questionnaire. We analysed the data using a Cox regression model with time-varying covariates. An interaction term between PM2.5 and physical activity was included to examine the overall interaction effects.
Results
Compared with high physical activity, moderate and inactive/low physical activity were associated with a higher risk of diabetes (HR [95% CI] 1.31 [1.22, 1.41] and 1.56 [1.46, 1.68], respectively). Participants with moderate/high PM2.5 had a higher risk of type 2 diabetes than the participants exposed to low PM2.5 (HR 1.31 [1.22, 1.40] and 1.94 [1.76, 2.14], respectively). The participants with high physical activity and low PM2.5 had a 64% lower risk of type 2 diabetes than those with inactive/low physical activity and high PM2.5.
Conclusions/interpretation
Higher physical activity and lower PM2.5 exposure are associated with lower risk of type 2 diabetes. Habitual physical activity can reduce the risk of diabetes regardless of the levels of PM2.5 exposure. Our results indicate that habitual physical activity is a safe diabetes prevention strategy for people residing in relatively polluted regions.
Graphical abstract

Similar content being viewed by others

Introduction
Type 2 diabetes is a global public health challenge that poses an overwhelming burden on healthcare systems. In 2019, 463 million adults were living with diabetes worldwide and 90% of them had type 2 diabetes [1]. It is estimated that diabetes contributed to 4.2 million deaths and US$760 billion in health expenditure [1]. Habitual physical activity is an effective way to prevent type 2 diabetes [2, 3] and other non-communicable chronic diseases that lead to premature deaths including CVD, cancer and respiratory diseases [4]. WHO recommends that adults should take at least 150 min of moderate physical activity each week, but approximately 31% of the world’s population fails to meet this recommendation [5]. To reduce the health burden of non-communicable diseases, including diabetes, WHO has proposed to reduce insufficient physical activity by 10% by 2025 in WHO Member States [5].
However, physical activity increases the inhalation of air pollutants due to higher ventilation, which may exacerbate the adverse health effects of air pollution. An increasing body of evidence has shown that air pollution is a novel risk factor for the development of type 2 diabetes [6, 7]. Thus, the risk–benefit relationship between air pollution and physical activity has become an important public concern as more than 91% of the world’s population lives in a place where air quality does not meet the WHO guidelines [8]. There is limited information on the combined associations of air pollution and habitual physical activity with the development of type 2 diabetes [9]. Health guidelines are urgently needed, especially in regions with significant air pollution, to inform people whether they can benefit from habitual physical activity. We therefore investigated the combined associations of habitual physical activity and chronic exposure to ambient particulate matter with an aerodynamic diameter less than 2.5 μm (PM2.5) with the incidence of type 2 diabetes in a longitudinal cohort of 156,314 adults who had 422,831 medical examinations in Taiwan, where the annual PM2.5 concentrations exceed the limit recommended by the WHO guidelines.
Methods
Study design and participants
This study was based on an ongoing large prospective cohort in Taiwan. Details of this cohort have been described [10,11,12]. In brief, this is an open and dynamic cohort established in 1994 by MJ Health Management Institution that provides a standard medical health screening programme for Taiwan residents through a paid membership. All participants visit the institute and undergo a series of medical examinations including anthropometric measurements, physical examinations, blood and urinary tests, and a standard self-administered questionnaire survey. Participants are encouraged to visit the institute regularly and undergo medical examinations. The data have been stored electronically since 1996, and more than 600,000 participants were recruited between 1996 and 2016. Written informed consent was given by each participant prior to each medical examination. Ethical approval for this study was acquired from the Joint Chinese University of Hong Kong - New Territories East Cluster Clinical Research Ethics Committee.
Electronic supplementary material (ESM) Fig. 1 shows the participant selection. We selected adults aged ≥18 years as we were targeting type 2 diabetes. A total of 435,529 participants with plasma glucose measurements were recruited between 2001 and 2016, where the PM2.5 data were available. A total of 67,650 participants were excluded due to incomplete information (2953 on PM2.5 because of missing address, 28,786 on habitual physical activity and 35,911 on the covariates). Compared with the 367,879 participants included in the preliminary sample, the 67,650 participants excluded due to missing data had similar distributions in age (mean: 42.1 years vs 41.9 years), sex (men: 44.6% vs 50.5%), smoking status (never smokers: 74.3% vs 75.4%), BMI (23.2 kg/m2 vs 23.2 kg/m2), PM2.5 concentration (mean: 24.9 μg/m3 vs 26.2 μg/m3), and physical activity (high physical activity: 37.1% vs 34.8%), but they were slightly less educated (college or above: 54.1% vs 67.3%).
We further excluded 205,740 participants who had only one medical examination and 5825 participants who had diabetes at baseline. Finally, 156,314 participants with 422,831 medical examinations were included in the data analysis. Compared with the included participants for data analysis, the 205,740 excluded participants with only one medical examination generally had similar distributions in the characteristics except they had a slightly higher level of physical activity (ESM Table 1). The numbers of participants and observations included in this data analysis differ slightly from those in our previous study [7] as we have recently updated the data up to December 2016, and the covariates selected in this data analysis are slightly different.
Habitual physical activity assessment
Detailed information on the assessment of habitual physical activity has been described in previous publications [2, 10, 13]. In brief, we collected information on habitual physical activity based on the standardised self-administered questionnaire. Each participant was requested to report details (including intensity and duration) of weekly habitual physical activity during the month prior to the medical examination. The intensity was classified into the following four levels: light (e.g. walking), moderate (e.g. brisk walking), medium-vigorous (e.g. jogging) and high-vigorous (e.g. running). We then assigned each intensity level a specific metabolic equivalent (MET: 1 MET = 1 kJ h−1 [kg bodyweight]−1) of 10.5, 18.8, 27.2 and 35.6, respectively, based on the compendium of physical activities and a previous study [10, 14]. The weekly total time spent on physical activity was also collected. Thereafter, the product of physical activity intensity (MET) and duration (hours) was calculated as the volume of weekly MET-h. If the participants performed multiple types of exercises with two or more intensity categories, weighted MET values were calculated according to the time spent on each type of exercise. Finally, the participants were broadly grouped into three categories based on the tertile cut-off values of the weekly MET-h: inactive/low physical activity (0.0–0.6), moderate physical activity (0.6–9.8) and high physical activity (>9.8). Because most participants had 0.0 MET-h in the inactive/low physical activity category and previous studies showed a non-linear association between physical activity and non-communicable diseases [15, 16], the categorical physical activity variable was used.
Particulate matter exposure assessment
Details regarding ambient PM2.5 assessment were described in previous studies [7, 17,18,19]. Briefly, the ground-level concentration of PM2.5 was estimated based on a spatiotemporal model which was developed using the Aerosol optical depth (AOD) data derived from the Moderate Resolution Imaging Spectroradiometer (MODIS) installed in US National Aeronautics and Space Administration satellites. The AOD data obtained had a resolution of 1 × 1 km2, and the sample size was comparable to the general worldwide level. Ground-level ambient PM2.5 from >70 monitoring stations in Taiwan (2005–2014) were used to validate the model. The correlation coefficients for the association between the measurements from satellite and ground-monitoring stations ranged from 0.72 to 0.83 between 2005 and 2014.
Each participant’s address was geocoded into latitude and longitude data. The address information was routinely updated during each medical visit. Thus, changes of address were recorded and taken into account in the data analysis. We assigned estimated ambient PM2.5 concentrations to each participant based on the geocoded latitude and longitude. Because type 2 diabetes is a chronic disease, we used 2 year mean PM2.5 concentration (the year of the visit and the year before the visit) as the indicator for chronic PM2.5 exposure.
We classified the participants into quartiles or deciles in our previous study on the association between PM2.5 and incident diabetes [7]. However, to correspond to the categories of physical activity in this study, we classified the participants into three categories based on the tertile cut-off values of PM2.5. Both categorical PM2.5 and continuous PM2.5 (every 10 μg/m3) were used for data analysis.
Outcome ascertainment
Incident type 2 diabetes was the outcome in this study and was defined as in our previous study [7]. An overnight-fasting blood sample was taken in the morning from each participant. A Hitachi 7150 analyser (Tokyo, Japan) was used to measure fasting plasma glucose (FPG) level enzymatically until 2005, after which a Toshiba C8000 analyser (Tokyo, Japan) was used. After the baseline assessment at the first visit, all 156,314 non-diabetic participants were followed up, and those with type 2 diabetes were identified by medical assessment (defined as FPG ≥7 mmol/l, or self-reported physician-diagnosed diabetes) in subsequent visits. The endpoint was the first occurrence of type 2 diabetes or the last visit if type 2 diabetes did not occur.
Covariates
The covariates were selected a priori, mainly based on literature review [20, 21]. Details of covariates data collection have been described in the Technical Reports of MJ Health Research Foundation [12] and other publications [7, 11, 22]. Information on demographics, socioeconomic status, lifestyle and medical history was collected using standard questionnaires. In addition to habitual physical activity, the participants were asked to report their intensity of physical activity at work according to the levels of exertion described in the questionnaire: mostly sedentary (e.g. clerk), combination of sitting/standing/walking (e.g. nurse), mostly standing or walking (e.g. retail salesperson) or hard labour (e.g. porter). The variable ‘physical activity at work’ was included as a covariate. BMI was calculated using weight and height, which were measured with the participants wearing light indoor clothing without shoes. Seated BP was measured by auto-sphygmomanometer (Citizen CH-5000, Tokyo, Japan). An overnight-fasting blood sample was taken in the morning to measure the lipid profile using a Hitachi 7150 (until 2005) or Toshiba C8000 device (after 2005).
The covariates included in the present study are: sex (male or female); age (years); education (less than high school [<10 years], high school [10–12 years], college or university [13–16 years] or postgraduate [>16 years]); smoking status (never, former or current); alcohol consumption (never/seldom [drank less than once a week], former [drank at least once a week but quit later] or current [drank more than once a week]); physical activity at work (mostly sedentary, combination of sitting/standing/walking, mostly standing or walking, or hard labour); vegetable intake (seldom [<1 serving/day], moderate [1–2 servings/day], or frequent [>2 servings/day]); fruit intake (seldom [<1 serving/day], moderate [1–2 servings/day], or frequent [>2 servings/day]); occupational exposure (exposure to dust or solvent: yes or no); BMI (kg/m2); hypertension (systolic BP ≥ 140 mmHg, diastolic BP ≥ 90 mmHg or self-reported physician-diagnosed hypertension: yes or no); dyslipidaemia (total cholesterol ≥13.4 mmol/l, triacylglycerol ≥11.2 mmol/l or HDL-cholesterol <2.2 mmol/l: yes or no); self-reported physician-diagnosed CVD (yes or no) and cancer (yes or no); baseline glucose (mmol/l); season (spring [March–May], summer [June–August], autumn [September–November], or winter [December–February]); and year of enrolment.
Data analysis
Cox regression model with time-varying covariates was used to investigate the combined associations of chronic PM2.5 exposure and habitual physical activity with the development of type 2 diabetes. All covariates except for sex and baseline glucose were treated as time-varying variables in the data analysis to account for the changes in these covariates during the study period. Follow-up time was used as the timescale. A city-level random intercept was included to control for clustering effects within the same city. Sixteen municipalities or cities were included: Taipei, Keelung, Taoyuan, Hsinchu, Yilan, Miaoli, Taichung, Changhua, Nantou, Hualien, Yunlin, Chiayi, Tainan, Kaohsiung, Taitung and Pingtung. Three models with different covariate combinations were developed to assess the main associations of incident diabetes with long-term PM2.5 exposure and habitual physical activity separately: Model 1 was adjusted for demographic factors (age, sex and educational level); Model 2 was further adjusted for baseline glucose, lifestyle factors (smoking, alcohol consumption, vegetable intake, fruit intake, occupational exposure and physical labour at work), season and year of enrolment; and Model 3 was adjusted for the covariates in Model 2 plus diabetes-related health factors (BMI, hypertension, dyslipidaemia, self-reported physician-diagnosed CVD and cancer). Natural cubic spline function was used to draw the concentration–response curve of the association between PM2.5 and incident diabetes adjusting for Model 3’s covariates. An interaction term ‘continuous PM2.5 (every 10 μg/m3) × category of physical activity’ was included in Model 3 to explore the potential interactions.
Subgroup analyses were performed stratified by the categories of PM2.5 and habitual physical activity separately to examine the associations with PM2.5 or habitual physical activity in each stratum. Finally, the participants were classified into nine groups according to the categories of PM2.5 and physical activity with reference to the participants with inactive/low physical activity and high PM2.5 exposure.
To examine the robustness of the estimates, a series of sensitivity analyses were conducted: (1) we excluded participants who had self-reported physician-diagnosed CVD or cancer at baseline to eliminate the potential comorbidity effects; (2) we used annual PM2.5 exposure in the year previous to the medical examination to examine the stability of the associations; (3) we only included participants with at least 2 years of follow-up because the development of type 2 diabetes is a chronic process; (4) we only included participants enrolled until 2005 with FPG and lipid measurements obtained using the Hitachi 7150 or those enrolled since 2005 with FPG and lipid measurements obtained using the Toshiba C8000 to eliminate potential equipment-related measurement bias; (5) we only included participants older than 30 years of age to better differentiate type 1 and type 2 diabetes; (6) we conducted data analysis for men and women separately to compare the associations within the sexes; and (7) we conducted data analysis for never and ever smokers separately to compare the associations in different smoking status.
The statistical analyses were performed using R 3.6.1. (R Development Core Team, Vienna, Austria) with the ‘coxme’ package. A two-tailed p value of less than 0.05 was considered statistically significant.
Results
Table 1 shows the general characteristics of the 156,314 participants with 422,831 medical examinations included in the main analysis. The participants were generally well educated and had a relatively low prevalence of smoking and alcohol consumption. More than 66% of the participants had sedentary jobs. The PM2.5 concentrations were generally comparable among the participants with different categories of habitual physical activity. The mean duration of follow-up was 5.2 years with an SD of 3.6 years. A total of 5305 new cases of type 2 diabetes were identified with an incidence rate of 6.53 per 1000 person-years. The participants underwent a median of three medical examinations (range, 2–26). The median examination interval was 16 months (IQR 12–26 months).
Figure 1 shows the distributions of PM2.5 concentrations by year. The contrast in PM2.5 exposure was large (range 6.38–49.78 μg/m3; overall IQR 21.80–28.07 μg/m3). The PM2.5 concentration peaked in 2004 and has gradually decreased ever since.
Temporal distribution of the 2 year mean of PM2.5 concentrations by year in Taiwan. Boxes cover the IQR with centre lines referring to the median value. Whiskers extend to the highest observations within three IQRs of the box, with more extreme observations shown as circles. This represents the distribution of the 156,314 participants at baseline
Table 2 shows the main associations of incident diabetes with habitual physical activity and chronic PM2.5 exposure. High habitual physical activity was associated with a lower incidence of type 2 diabetes. In contrast, a high level of chronic PM2.5 exposure was associated with a higher incidence of type 2 diabetes. Mutual adjustment generally resulted in slight changes in the associations. The concentration–response curve for the association between PM2.5 and the incidence of type 2 diabetes is presented in ESM Fig. 2. Overall, the concentration–response curve was approximately linear (likelihood ratio test: χ2 = 2.8, p = 0.09). No significant interactions were observed (p = 0.52).
Table 3 shows the results of the subgroup analysis. Similar associations were observed (i.e. high habitual physical activity was associated with a lower incidence of type 2 diabetes in each PM2.5 stratum, whereas high level of PM2.5 exposure was associated with a higher incidence of type 2 diabetes in each physical activity stratum).
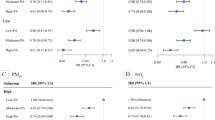
Figure 2 shows the combined associations of habitual physical activity and chronic PM2.5 exposure with the development of type 2 diabetes. The participants with a high physical activity and a low PM2.5 exposure had the lowest risk of developing type 2 diabetes, whereas inactive/low physical activity participants with a high PM2.5 exposure had the highest risk of developing type 2 diabetes. The corresponding estimated HRs are shown in ESM Table 2.
Combined associations of habitual physical activity and chronic PM2.5 exposure with diabetes in adults in Taiwan. The results were fully adjusted for age, sex, educational level, physical labour at work, smoking, drinking, vegetable intake, fruit intake, occupational exposure, baseline glucose, BMI, hypertension, dyslipidaemia, self-reported physician-diagnosed CVD and cancer, season and year of enrolment. Combined associations with participants classified into nine groups according to PM2.5 and physical activity (PA) categories
Sensitivity analyses generally yielded consistent results (ESM Tables 3–8). The associations of incident diabetes with PM2.5 and physical activity were relatively stronger for the participants enrolled after 2005 (ESM Table 9), as compared with the associations for those enrolled before 2005 (ESM Table 10).
Discussion
To the best of our knowledge, this is the first longitudinal cohort study that investigated the combined effects of habitual physical activity and chronic exposure to PM2.5 on the development of type 2 diabetes in the general population. In this Asian population with a mean PM2.5 exposure of 26.1 μg/m3, we found that high levels of habitual physical activity combined with low levels of chronic PM2.5 exposure were associated with a lower risk of developing type 2 diabetes, whereas low levels of habitual physical activity combined with high levels of chronic PM2.5 exposure were associated with a higher risk of developing type 2 diabetes. The benefits of habitual physical activity on type 2 diabetes remained stable in participants with different levels of PM2.5 exposure. The adverse associations of type 2 diabetes with chronic PM2.5 exposure were also observed in participants with various levels of habitual physical activity. No significant interaction was observed between PM2.5 and physical activity on type 2 diabetes development.
Comparison with previous studies
It is well known that habitual physical activity may prevent the development of diabetes and improve the disease prognosis. Our results show that habitual physical activity was associated with a lower incidence of type 2 diabetes, which is in line with previous studies [2, 3]. Our results are also in line with many previous studies that reported a positive association between air pollution and type 2 diabetes [6, 7].
Compared with our previous study based on the same cohort [7]. the effect sizes of PM2.5 in this study were slightly higher. It is possible that our previous study did not consider the city-level random effects. A study has shown that city-level random effects may affect the associations between air pollution and mortality rate [23], but we were not aware of this at that time. The concentration–response trend was also smoother after taking into account the city-level random effects in the data analysis (ESM Fig. 2).
There is limited information on the combined associations of habitual physical activity and chronic PM2.5 exposure with the development of type 2 diabetes and our study provides novel insights on this topic. We observed benefits of physical activity for diabetes regardless of the levels of PM2.5 exposure. A study in Korea, which targeted an older population (≥58 years of age), yielded similar conclusions [9]. Our finding is also in line with previous studies that indicated that the benefits of physical activity outweighed the adverse health effects of air pollution for some other outcomes including blood pressure [24], systemic inflammation [25], lung function or respiratory diseases [26, 27], recurrent myocardial infarction [28], and mortality rate [29, 30]. However, a few studies showed a significant interaction of air pollution and physical activity and reported that air pollution counteracted the benefits of physical activity [11, 31, 32]. The discrepancy may be due to a number of factors such as health outcomes, study designs, sample size, study duration and the level of air pollution in the study region.
Although the measurements of physical activity and PM2.5 levels were not comparable (i.e. physical activity was measured in MET-h, whereas PM2.5 was measured in μg/m3), our analyses based on the tertile categories suggest that the PM2.5–diabetes associations were slightly stronger than the physical activity–diabetes associations (i.e. inactive/low physical activity was associated with a 56% higher risk of diabetes, whereas high PM2.5 exposure was associated with a 94% higher risk of diabetes; see Model 3 in Table 2). This finding is in line with our previous studies which also indicated that PM2.5 had a stronger association with hypertension and lung function than physical activity [11, 33], suggesting the importance of air pollution mitigation in the prevention of diabetes.
Potential mechanism
Several hypotheses have been proposed regarding the protective effects of physical activity against type 2 diabetes, including the maintenance of appropriate body weight, improvement of insulin sensitivity, alleviation of systemic inflammation and promotion of lipid regulation [34,35,36]. The biological mechanism that underlies the association between chronic PM2.5 exposure and type 2 diabetes is not completely understood. Previous animal experiments showed that PM2.5 exposure was associated with a higher level of systemic inflammation and oxidative stress [37]. The study by Houstis et al. indicated that the increase of reactive oxygen species in the two constructed cellular models may lead to insulin resistance, which is a cardinal feature of type 2 diabetes [38]. In addition, PM2.5 pollution was associated with central nervous system inflammation and abnormal activation of the sympathetic nervous system [39]. Dysfunction of the autonomic nervous system was proved to be associated with early glucose dysmetabolism and the development of diabetes [40]. Therefore, dysfunction of the autonomic nervous system could be another potential pathway for the PM2.5–diabetes association.
The manner in which habitual physical activity and chronic PM2.5 exposure jointly affect the development of type 2 diabetes remains unclear. A previous study showed that the additional air pollutants inhaled while performing physical activity accounted for only a small fraction of the total inhaled air pollutants [41]. This may partly explain the positive associations between physical activity and type 2 diabetes regardless of the levels of PM2.5 exposure. Moreover, the long-term benefits of habitual physical activity may reverse the acute adverse effects associated with the additional intake of air pollutants during exercise [29].
Strengths and limitations
This study has several important strengths. First, its large sample size enabled us to conduct a series of subgroup and sensitivity analyses to test the robustness of the associations. The large sample size also gave us sufficient statistical power to obtain stable and precise estimates. Second, the longitudinal nature of this study allowed us to consider changes in physical activity habits and chronic PM2.5 exposure over the study period. Third, we collected comprehensive information on physical activity (including household work, transportation, leisure-time physical activity and daily work) and a number of covariates. Finally, this study was conducted in an Asian population with a relatively high level of air pollution exposure (approximately 2.6 times the PM2.5 limit recommended by WHO guidelines). The research findings have valuable regulatory and policy implications for both air pollution and physical activity guidelines in countries/regions with similar levels of pollution.
Several limitations should be noted. First, we did not distinguish whether the participants’ habitual physical activity was performed indoors or outdoors; however, a 2017 national survey showed that 92.7% of Taiwanese residents reported outdoor exercise as their most frequent physical activity [42]. Also, habitual physical activity was assessed with a self-administered questionnaire (the content validity and reliability have been reported in a previous study) [10]. Second, it is difficult to distinguish between type 1 and type 2 diabetes in a large-scale epidemiological study. However, we only selected participants without diabetes who were at least 18 years of age at baseline, so most cases of incident diabetes in our study should be type 2 diabetes. In addition, the sensitivity analysis that excluded the participants with a baseline age of <30 years yielded similar results. Third, the spatiotemporal model used for PM2.5 assessment was not validated before 2005 due to the limited number of monitoring stations in Taiwan between 2000 and 2004 [19, 43]. However, this is unlikely to have affected the PM2.5 estimates in the period of 2000–2004 as the PM2.5 was estimated by the spatiotemporal model based on satellite data rather than the data from monitoring stations. There is no larger amount of missing satellite data in this period. In addition, we estimated PM2.5 exposure at fixed addresses of the participants. Although the resolution of 1 × 1 km2 in our study was relatively high compared with other studies, PM2.5 may still vary significantly within a 1 × 1 km2 grid, especially in some urban areas with heavy traffic and tall buildings. More advanced technologies are needed for more accurate assessment of exposure in future studies. Finally, our study was conducted in an area with moderate pollution. Further studies in areas with more severe air pollution are required to examine the applicability of our findings. The participants in this study were relatively well educated and healthy. Therefore, generalisation of the findings to other populations should be conducted with caution.
In conclusion, high habitual physical activity combined with low PM2.5 exposure are associated with a lower risk of developing type 2 diabetes, whereas low physical activity combined with high PM2.5 exposure are associated with a higher risk of developing type 2 diabetes. Habitual physical activity reduces the risk of diabetes regardless of the level of PM2.5 exposure, and PM2.5 exposure generally increases the risk of diabetes regardless of the level of habitual physical activity. Our findings suggest that habitual physical activity is a safe strategy for diabetes prevention for people who reside in relatively polluted areas and should be promoted. Our study reinforces the importance of air pollution mitigation for diabetes prevention.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available for data protection reasons but are available from MJ Health Research Foundation.
Abbreviations
- AOD:
-
Aerosol optical depth
- FPG:
-
Fasting plasma glucose
- MET:
-
Metabolic equivalents
- PM2.5 :
-
Particulate matter with an aerodynamic diameter less than 2.5 μm
References
Saeedi P, Petersohn I, Salpea P et al (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res Clin Pract 157:107843. https://doi.org/10.1016/j.diabres.2019.107843
Lao XQ, Deng HB, Liu XD et al (2019) Increased leisure-time physical activity associated with lower onset of diabetes in 44828 adults with impaired fasting glucose: A population-based prospective cohort study. Br J Sport Med 53(14):895–900. https://doi.org/10.1136/bjsports-2017-098199
Colberg SR, Sigal RJ, Yardley JE et al (2016) Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 39(11):2065–2079. https://doi.org/10.2337/dc16-1728
Forouzanfar MH, Afshin A, Alexander LT et al (2016) Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 388(10053):1659–1724. https://doi.org/10.1016/S0140-6736(16)31679-8
World Health Organization. Noncommunicable diseases - physical activity. Available at http://www.emro.who.int/noncommunicable-diseases/causes/physical-inactivity.html. Last access on June 20, 2020
Yang BY et al (2020) Ambient air pollution and diabetes: A systematic review and meta-analysis. Environ Res 180:108817. https://doi.org/10.1016/j.envres.2019.108817
Lao XQ, Guo C, Chang LY et al (2019) Long-term exposure to ambient fine particulate matter (PM2.5) and incident type 2 diabetes: A longitudinal cohort study. Diabetologia 62(5):759–769. https://doi.org/10.1007/s00125-019-4825-1
World Health Organization (2018) 9 out of 10 people worldwide breathe polluted air, but more countries are taking action. Available from www.who.int/news-room/detail/02-05-2018-9-out-of-10-people-worldwide-breathe-polluted-air-but-more-countries-are-taking-action. Accessed 20 Jun 2020
Kim SR, Choi D, Choi S et al (2020) Association of combined effects of physical activity and air pollution with diabetes in older adults. Environ Int 145:106161. https://doi.org/10.1016/j.envint.2020.106161
Wen CP, Wai JP, Tsai MK et al (2011) Minimum amount of physical activity for reduced mortality and extended life expectancy: A prospective cohort study. Lancet 378(9798):1244–1253. https://doi.org/10.1016/S0140-6736(11)60749-6
Guo C, Bo Y, Chan TC et al (2020) Does fine particulate matter (PM2.5) affect the benefits of habitual physical activity on lung function in adults: A longitudinal cohort study. BMC Med 18(1):134
MJ Health Research Foundation, MJ Health Resource Centre (2016) MJ Health Database (MJHD): Technical Report, MJHRF-TR-01. Available from www.mjhrf.org/file/en/report/mjhrf-tr-01mjhealthdatabase.Pdf. Accessed 18 Jan 2021
Guo C, Tam T, Bo Y, Chang LY, Lao XQ, Thomas GN (2020) Habitual physical activity, renal function and chronic kidney disease: A cohort study of nearly 200 000 adults. Br J Sports Med 54(20):1225–1230. https://doi.org/10.1136/bjsports-2019-100989
Ainsworth BE, Haskell WL, Whitt MC et al (2000) Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exerc 32(9 Suppl):S498–S504. https://doi.org/10.1097/00005768-200009001-00009
Egan BM (2017) Physical activity and hypertension knowing is not enough; we must apply. Willing is not enough; we must do-von Goethe. Hypertension 69(3):404–406. https://doi.org/10.1161/HYPERTENSIONAHA.116.08508
Andersen K, Mariosa D, Adami HO et al (2014) Dose-response relationship of total and leisure time physical activity to risk of heart failure: A prospective cohort study. Circ Heart Fail 7(5):701–708. https://doi.org/10.1161/CIRCHEARTFAILURE.113.001010
Lin CQ, Li Y, Yuan ZB, Lau AKH, Li CC, Fung JCH (2015) Using satellite remote sensing data to estimate the high-resolution distribution of ground-level PM2.5. Remote Sens Environ 156:117–128. https://doi.org/10.1016/j.rse.2014.09.015
Lin CQ, Liu G, Lau AKH et al (2018) High-resolution satellite remote sensing of provincial PM2.5 trends in China from 2001 to 2015. Atmos Environ 180:110–116. https://doi.org/10.1016/j.atmosenv.2018.02.045
Zhang Z, Chang LY, Lau AKH et al (2017) Satellite-based estimates of long-term exposure to fine particulate matter are associated with C-reactive protein in 30 034 Taiwanese adults. Int J Epidemiol 46(4):1126–1136. https://doi.org/10.1093/ije/dyx069
Zimmet P, Alberti K, Shaw J (2001) Global and societal implications of the diabetes epidemic. Nature 414(6865):782–787. https://doi.org/10.1038/414782a
Puett RC, Hart JE, Schwartz J, Hu FB, Liese AD, Laden F (2011) Are particulate matter exposures associated with risk of type 2 diabetes? Environ Health Perspect 119(3):384–389. https://doi.org/10.1289/ehp.1002344
Guo C, Zhang ZL, Lau AKH et al (2018) Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: A longitudinal, cohort study. Lancet Planet Health 2(3):E114–EE25
Beelen R, Raaschou-Nielsen O, Stafoggia M et al (2014) Effects of long-term exposure to air pollution on natural-cause mortality: An analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet 383(9919):785–795. https://doi.org/10.1016/S0140-6736(13)62158-3
Avila-Palencia I, Laeremans M, Hoffmann B et al (2019) Effects of physical activity and air pollution on blood pressure. Environ Res 173:387–396. https://doi.org/10.1016/j.envres.2019.03.032
Zhang ZL, Hoek G, Chang LY et al (2018) Particulate matter air pollution, physical activity and systemic inflammation in Taiwanese adults. Int J Hyg Environ Health 221(1):41–47. https://doi.org/10.1016/j.ijheh.2017.10.001
Fuertes E, Markevych I, Jarvis D et al (2018) Residential air pollution does not modify the positive association between physical activity and lung function in current smokers in the ECRHS study. Environ Int 120:364–372. https://doi.org/10.1016/j.envint.2018.07.032
Fisher JE, Loft S, Ulrik CS et al (2016) Physical activity, air pollution, and the risk of asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 194(7):855–865. https://doi.org/10.1164/rccm.201510-2036OC
Kubesch NJ, Therming Jørgensen J, Hoffmann B et al (2018) Effects of leisure-time and transport-related physical activities on the risk of incident and recurrent myocardial infarction and interaction with traffic-related air pollution: A cohort study. J Am Heart Assoc 7(15):e009554
Andersen ZJ, de Nazelle A, Mendez MA et al (2015) A study of the combined effects of physical activity and air pollution on mortality in elderly urban residents: The Danish Diet, Cancer, and Health Cohort. Environ Health Perspect 123(6):557–563. https://doi.org/10.1289/ehp.1408698
Sun S, Cao W, Qiu H et al (2020) Benefits of physical activity not affected by air pollution: A prospective cohort study. Int J Epidemiol 49(1):142–152. https://doi.org/10.1093/ije/dyz184
McConnell R, Berhane K, Gilliland F et al (2002) Asthma in exercising children exposed to ozone: A cohort study. Lancet 359(9304):386–391. https://doi.org/10.1016/S0140-6736(02)07597-9
Endes S, Schaffner E, Caviezel S et al (2017) Is physical activity a modifier of the association between air pollution and arterial stiffness in older adults: The SAPALDIA cohort study. Int J Hyg Environ Health 220(6):1030–1038. https://doi.org/10.1016/j.ijheh.2017.06.001
Guo C, Zeng Y, Chang L et al (2020) Independent and opposing associations of habitual exercise and chronic PM2.5 exposures on hypertension incidence. Circulation 142(7):645–656. https://doi.org/10.1161/CIRCULATIONAHA.120.045915
Bassuk SS, Manson JE (2005) Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol 99(3):1193–1204. https://doi.org/10.1152/japplphysiol.00160.2005
Pedersen BK (2017) Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur J Clin Investig 47(8):600–611. https://doi.org/10.1111/eci.12781
Lao XQ, Thomas GN, Jiang CQ et al (2007) C-reactive protein and the metabolic syndrome in older Chinese: Guangzhou Biobank Cohort Study. Atherosclerosis 194(2):483–489. https://doi.org/10.1016/j.atherosclerosis.2006.08.061
Sun Q, Yue P, Deiuliis JA et al (2009) Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 119(4):538–546. https://doi.org/10.1161/CIRCULATIONAHA.108.799015
Houstis N, Rosen ED, Lander ES (2006) Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature 440(7086):944–948. https://doi.org/10.1038/nature04634
Ying Z, Xu X, Bai Y et al (2014) Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: A role for hypothalamic inflammation. Environ Health Perspect 122(1):79–86. https://doi.org/10.1289/ehp.1307151
Carnethon MR, Jacobs DR, Sidney S, Liu K (2003) Influence of autonomic nervous system dysfunction on the development of type 2 diabetes: The CARDIA study. Diabetes Care 26(11):3035–3041. https://doi.org/10.2337/diacare.26.11.3035
Rojas-Rueda D, de Nazelle A, Tainio M, Nieuwenhuijsen MJ (2011) The health risks and benefits of cycling in urban environments compared with car use: health impact assessment study. BMJ 343 https://doi.org/10.1136/bmj.d4521
Department of Physical Education Ministry of Education (2017) Report of Active Cities, Taiwan. 2017. Available from https://isports.sa.gov.tw/Index.aspx. Accessed 20 June 2020
Lao XQ, Zhang Z, Lau AK et al (2018) Exposure to ambient fine particulate matter and semen quality in Taiwan. Occup Environ Med 75(2):148–154. https://doi.org/10.1136/oemed-2017-104529
Acknowledgements
We would like to thank MJ Health Research Foundation for authorising the use of MJ health data (authorisation code: MJHR2019006A). Any interpretation or conclusion related to this manuscript does not represent the views of MJ Health Research Foundation. We also appreciate the contributions of the editors and reviewers for their valuable and constructive comments, which helped us improve our manuscript substantially.
Author’s relationships and activities
Any interpretation or conclusion related to this manuscript is solely that of the authors and does not represent the views of the MJ Health Research Foundation. The authors declare that there are no relationships or activities that might bias, or be perceived to bias their work.
Funding
This work was supported by RGC General Research Fund (14603019) and Environmental Health Research Fund of the Chinese University of Hong Kong (7104946). CG is in part supported by the Faculty Postdoctoral Fellowship Scheme of the Faculty of Medicine of the Chinese University of Hong Kong. YB and YZ are supported by the PhD Studentship of the Chinese University of Hong Kong.
Author information
Authors and Affiliations
Contributions
XQL conceived and designed the study. LC, AKHL, CL, TT and XQL acquired the data. CG, HTY, YB and YZ searched the literature. CG, HTY, GH and XQL analysed and interpreted the data. CG, HTY, GH and XQL drafted the manuscript. All authors contributed to study conception and design, revised the manuscript critically for important intellectual content, and approved the final version to be published. XQL obtained the funding. LC, AKHL, GH, TT and XQL supervised this study. XQL is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cui Guo and Hsiao Ting Yang are joint first authors
Supplementary Information
ESM
(PDF 598 kb)
Rights and permissions
About this article
Cite this article
Guo, C., Yang, H.T., Chang, Ly. et al. Habitual exercise is associated with reduced risk of diabetes regardless of air pollution: a longitudinal cohort study. Diabetologia 64, 1298–1308 (2021). https://doi.org/10.1007/s00125-021-05408-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-021-05408-4