Abstract
Aims/hypothesis
We assessed the contribution of glucagon-like peptide-1 (GLP-1) receptor (GLP-1R) signalling to thermogenesis induced by high-fat diet (HFD) consumption. Furthermore, we determined whether brown adipose tissue (BAT) activity contributes to weight loss induced by chronic subcutaneous treatment with the GLP-1R agonist, liraglutide, in a model of diet-induced obesity.
Methods
Metabolic phenotyping was performed using indirect calorimetry in wild-type (WT) and Glp1r-knockout (KO) mice during chow and HFD feeding at room temperature and at thermoneutrality. In a separate study, we investigated the contribution of BAT thermogenic capacity to the weight lowering effect induced by GLP-1 mimetics by administering liraglutide (10 or 30 nmol kg−1 day−1 s.c.) to diet-induced obese (DIO) mice for 6 or 4 weeks, respectively. In both studies, animals were subjected to a noradrenaline (norepinephrine)-stimulated oxygen consumption \( \left(\overset{\cdot }{V}{\mathrm{O}}_2\right) \) test.
Results
At thermoneutrality, HFD-fed Glp1r-KO mice had similar energy expenditure (EE) compared with HFD-fed WT controls. However, HFD-fed Glp1r-KO mice exhibited relatively less EE when housed at a cooler standard room temperature, and had relatively lower \( \overset{\cdot }{V}{\mathrm{O}}_2 \) in response to a noradrenaline challenge, which is consistent with impaired BAT thermogenic capacity. In contrast to the loss of function model, chronic peripheral liraglutide treatment did not increase BAT activity as determined by noradrenaline-stimulated \( \overset{\cdot }{V}{\mathrm{O}}_2 \) and BAT gene expression.
Conclusions/interpretation
These data suggest that although endogenous GLP-1R signalling contributes to increased BAT thermogenesis, this mechanism does not play a significant role in the food intake-independent body weight lowering effect of the GLP-1 mimetic liraglutide in DIO mice.
Similar content being viewed by others
Introduction
Glucagon-like peptide-1 (GLP-1) receptor (GLP-1R) mimetics are effective therapeutics for type 2 diabetes mellitus (reviewed in [1]). In addition to experiencing improved glucose homeostasis, many patients lose weight during treatment with GLP-1-based therapies. Multiple clinical studies suggest that weight loss induced by GLP-1 is mainly attributed to reduced food intake (reviewed in [2]).
We and others have demonstrated that acute activation of central nervous system (CNS) GLP-1Rs increases the activity of brown adipose tissue (BAT) [3, 4]. Given the contribution of BAT to energy expenditure (EE), GLP-1-induced activation of BAT could potentially be an additional mechanism by which GLP-1R activation causes weight loss. BAT is a metabolically active tissue that oxidises, fuels and dissipates energy in the form of heat (reviewed in [5]). Animals lacking functional BAT have decreased EE and are more susceptible to developing obesity [6, 7]. High-fat diet (HFD) consumption increases thermogenesis and EE, which is referred to as diet-induced thermogenesis (DIT; reviewed in [8] and [9]). The mechanisms regulating DIT are not completely understood but seem to be partially due to increased sympathetic innervation of BAT [10, 11], which is assessed in animals at thermoneutral temperatures [7].
Recent studies have demonstrated the existence of functionally active BAT in adult humans [12–14], suggesting that agents that induce BAT thermogenesis may be attractive candidates for treating obesity [15]. Given the evidence of GLP-1R regulation of BAT as well as body weight (BW) loss induced by GLP-1R analogues, we aimed to investigate whether endogenous GLP-1R signalling contributes to increased BAT thermogenesis induced by high-fat feeding. Next, we aimed to determine whether BAT thermogenesis contributes to BW loss induced by chronic peripheral treatment with the GLP-1R agonist, liraglutide.
Methods
Animals
All studies were approved and performed following the guidelines of the institutional animal care and use committees of the University of Cincinnati. Glp1r-knockout (KO) and age-matched wild-type (WT) male mice on a C57/BL6J background were generated as previously described [16] and bred at the University of Cincinnati. Mice were maintained on a 12:12 h light–dark cycle at 22°C with free access to water and to either a standard chow control diet (CD; 5.6% fat; LM-485, Teklad, Harlan; Indianapolis, IN, USA) or a high-sucrose diet containing 58% energy from fat (HFD; Research Diets #D12331, New Brunswick, NJ, USA), as indicated. Mice were 12–14 weeks and 16–18 weeks of age for CD and HFD diet experiments, respectively.
Male C57BL/6J mice (The Jackson Laboratory; Bar Harbor, ME, USA) were fed HFD (Research Diets #D12331, New Brunswick) starting at 10 weeks of age and were then maintained on the diet for 24 weeks prior to liraglutide treatment. Mice were randomly assigned to groups using Microsoft Excel. Experimenters were not blind to group assignment and outcome assessment. All the data collected are presented. In the case of the gene expression analysis, a subset of samples per group was randomly chosen using Microsoft Excel.
Indirect calorimetry
Mice were housed in chambers with integrated control of ambient temperature and simultaneous measurement of food intake, locomotor activity and EE by indirect calorimetry (TSE Systems, Chesterfield, MO, USA). Mice were monitored at 22°C or 31°C to compare energy balance at standard room temperature or at thermoneutrality, respectively.
Noradrenaline (norepinephrine) stimulation of oxygen consumption
Animals were adapted to 31°C overnight (14–16 h) prior to experiments. Oxygen consumption \( \left(\overset{\cdot }{V}{\mathrm{O}}_2\right) \) was analysed in response to s.c. (1 mg kg−1) injection of noradrenaline (norepinephrine; Sigma-Aldrich, St Louis, MO, USA).
Body composition measurements
Whole-body composition (fat and lean mass) was measured using NMR technology (EchoMRI-100; Echomedical Systems, Houston, TX, USA) [17].
Chronic liraglutide treatment
Liraglutide was synthesised at Indiana University as previously described [18], and injected s.c. at a dose of 10 nmol kg−1 day−1 or 30 nmol kg−1 day−1 in diet-induced obese (DIO) mice. To determine the contribution of hypophagia to the BW lowering effect of liraglutide, a vehicle-treated pair-fed group was fed the same amount of food consumed by the corresponding liraglutide-treated group. Animals were housed in conventional cages at 22°C then placed in an indirect calorimetry system as indicated.
RNA extraction and quantitative PCR
RNA from interscapular BAT (iBAT), inguinal white adipose tissue (iWAT), quadriceps muscle and soleus muscle was extracted using a commercially available kit (RNeasy; Qiagen, Valencia, CA, USA) following the manufacturer’s instructions. After DNase I treatment (Invitrogen, Carlsbad, CA, USA), cDNA was synthesised using SuperScript-III (Invitrogen), and gene expression was determined by quantitative PCR using gene-specific TaqMan assays (Life Technologies, Carlsbad, CA, USA). Gene expression was evaluated using the ΔΔCt method. The housekeeping gene for iWAT and iBAT gene analysis was 18S, and that for muscle gene analysis was Rpl32.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA, USA). Statistical significance was determined by unpaired Student’s t test, one-way ANOVA followed by Tukey’s multiple comparison post hoc test or two-way ANOVA followed by Bonferroni’s multiple comparison post hoc test. All results are given as means ± SEM. Results were considered statistically significant when p < 0.05.
Results
Metabolic phenotype of Glp1r-KO mice at room temperature (22°C) and thermoneutrality (31°C) under chow-fed and HFD-fed conditions
First, we investigated the contribution of GLP-1R signalling in regulating energy balance in WT and Glp1r-KO mice fed a low-fat, CD at standard room temperature (22°C) or at thermoneutrality (31°C; Fig. 1a–g). By observing animals at both temperatures, we can dissect out differences in EE resulting from changes in BAT activity, given that this is greatly reduced at thermoneutral temperatures [19]. As we [3] and others [16] have previously reported, BW was similar between the WT and Glp1r-KO mice on a CD (Fig. 1a). Animals were placed in an indirect calorimetry system for 72 h to simultaneously assess \( \overset{\cdot }{V}{\mathrm{O}}_2 \), EE and food intake. Both genotypes had similar \( \overset{\cdot }{V}{\mathrm{O}}_2 \) at 22°C and 31°C (Fig. 1b, c). Average EE, food intake and net energy balance (calculated by subtracting caloric intake from EE) were significantly higher in animals maintained at an ambient temperature of 22°C vs 31°C, yet similar between genotypes at both temperatures (Fig. 1d–f). In contrast, locomotor activity was significantly lower at 22°C vs 31°C and significantly lower in Glp1r-KO compared with the WT controls (Fig. 1g). Overall, these data suggest that GLP-1R signalling does not play a major role in regulating energy metabolism in lean chow-fed mice.
Energy metabolism in CD-fed and (HFD)-fed WT and Glp1r-KO mice maintained at 22°C and 31°C. CD-fed WT (white bars, white squares) and Glp1r-KO (black bars, black squares) mice exhibited similar BW (a), \( \overset{\cdot }{V}{\mathrm{O}}_2 \) at 22°C (b) and \( \overset{\cdot }{V}{\mathrm{O}}_2 \) at 31°C (c). No differences between the genotypes were detected for average EE (d), food intake (e) or energy balance (f) at 22°C or 31°C, but Glp1r-KO mice exhibited significantly lower daily locomotor activity (g) (*p < 0.05; two-way ANOVA, main effect of genotype). Significantly different EE, food intake, energy balance and locomotor activity were observed in both genotypes at 31°C compared with 22°C (d–g; ***p < 0.001; two-way ANOVA, main effect of temperature). BW of HFD-fed Glp1r-KO (grey bars) mice was significantly lower than that of HFD-fed WT (white bars) mice (h; **p < 0.01; t test). When maintained at 22°C, Glp1r-KO mice (grey circles and bars) had lower \( \overset{\cdot }{V}{\mathrm{O}}_2 \) (i; p < 0.001; two-way ANOVA, main effect of genotype) and EE (k; † p < 0.05; two-way ANOVA with Bonferroni post hoc test) compared with WT controls (white circles and bars). When maintained at 31°C, both HFD-fed groups had a similar \( \overset{\cdot }{V}{\mathrm{O}}_2 \) (j) and EE (k). Food intake (l) and energy balance (m) in HFD-fed WT compared with Glp1r-KO mice were similar regardless of the ambient temperature. EE (k), food intake (l) and energy balance (m) were significantly lower in both groups at 31°C compared with 22°C (***p < 0.001; two-way ANOVA, main effect of temperature), while locomotor activity remained unchanged due to temperature (n). (n = 12)
Then, we aimed to determine whether endogenous GLP-1R signalling plays a role in regulating DIT. Therefore, a second group of mice was maintained on HFD for 4 weeks (Fig. 1h–n). Confirming previous observations [20, 21], the BW of HFD-fed Glp1r-KO mice was significantly lower than that of WT animals (Fig. 1h). \( \overset{\cdot }{V}{\mathrm{O}}_2 \) was significantly lower in HFD-fed Glp1r-KO mice compared with WT controls when animals were housed at 22°C (Fig. 1i). However, when HFD-fed animals were investigated at 31°C, to reduce BAT activity, \( \overset{\cdot }{V}{\mathrm{O}}_2 \) did not differ between the genotypes (Fig. 1j). Similarly, EE was significantly lower in Glp1r-KO mice compared with WT controls at 22°C, but no difference between the genotypes was observed at 31°C (Fig. 1k). Food intake and net energy balance did not differ between genotypes at either temperature, but was significantly lower in both groups at 31°C (Fig. 1l, m). Locomotor activity was similar between genotypes and remained unchanged regardless of the change in environmental temperature (Fig. 1n).
Noradrenaline-stimulated \( \overset{\cdot }{V}{\mathrm{O}}_2 \) in chow or HFD-fed Glp1r-KO mice
The different \( \overset{\cdot }{V}{\mathrm{O}}_2 \) at 22°C but not at 31°C, suggested a different contribution of BAT activity in the control of EE in HFD-fed WT and Glp1r-KO mice. To further evaluate the contribution of endogenous GLP-1R signalling to the BAT thermogenic capacity, we assessed the acute change in \( \overset{\cdot }{V}{\mathrm{O}}_2 \) in response to s.c. injection of noradrenaline in WT and Glp1r-KO animals that were previously housed overnight at thermoneutrality [19]. As expected, noradrenaline injection elicited a significant increase in \( \overset{\cdot }{V}{\mathrm{O}}_2 \) in CD-fed mice that was similar between both genotypes (Fig. 2a). The maximum \( \overset{\cdot }{V}{\mathrm{O}}_2 \) was similar in WT and Glp1r-KO animals, whereas AUC was slightly but significantly higher in CD-fed Glp1r-KO animals (Fig. 2b, c). In contrast, HFD-fed Glp1r-KO mice exhibited significantly reduced \( \overset{\cdot }{V}{\mathrm{O}}_2 \), maximum \( \overset{\cdot }{V}{\mathrm{O}}_2 \) as well as AUC \( \overset{\cdot }{V}{\mathrm{O}}_2 \) when compared with HFD-fed WT controls (Fig. 2d–f). These data suggest that BAT activity may be impaired due to lack of GLP-1R signalling under HFD-fed conditions.
Noradrenaline-stimulated \( \overset{\cdot }{V}{\mathrm{O}}_2 \) in WT and Glp1r-KO mice on a CD or HFD. \( \overset{\cdot }{V}{\mathrm{O}}_2 \) (a) and maximum \( \Delta \overset{\cdot }{V}{\mathrm{O}}_2 \) (b) following s.c. injection of noradrenaline was similar between Glp1r-KO (black circles) and WT (white circles) mice fed a chow diet. AUC of \( \Delta \overset{\cdot }{V}{\mathrm{O}}_2 \) was significantly higher in Glp1r-KO (black bars) mice compared with WT (white bars) mice (c; *p < 0.05, t test). In HFD-fed animals, \( \overset{\cdot }{V}{\mathrm{O}}_2 \) was significantly lower in Glp1r-KO (grey circles) compared with WT (white circles) mice following a noradrenaline injection (d; p < 0.001; two-way ANOVA, interaction time × genotype). Maximum \( \Delta \overset{\cdot }{V}{\mathrm{O}}_2 \) (e; **p < 0.01; t test) and AUC \( \Delta \overset{\cdot }{V}{\mathrm{O}}_2 \) (f; ***p < 0.001; t test) were lower in Glp1r-KO HFD-fed (grey bars) mice compared with WT HFD-fed (white bars) mice. (n = 12). Max., maximum
Food intake-independent effects of liraglutide on BW and fat mass
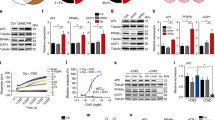
To determine whether chronic GLP-1R activation increases BAT activity, we treated DIO mice s.c. with 10 nmol kg−1 day−1 (Fig. 3a–c) or 30 nmol kg−1 day−1 of liraglutide (Fig. 3d–f) for 6 or 4 weeks, respectively. A pair-fed group was included in each study to determine food intake-independent effects of liraglutide. Mice treated with 10 nmol kg−1 day−1 had a transient reduction in food intake, which was similar in all groups at the end of the treatment period (Fig. 3a). Consistent with reduced feeding, BW and adiposity (Fig. 3b, c) in mice treated with 10 nmol kg−1 day−1 of liraglutide and those in the vehicle-PF group were significantly decreased compared with vehicle-treated animals fed ad libitum. However, BW and adiposity were significantly lower in 10 nmol kg−1 liraglutide-treated mice compared with vehicle-PF mice (Fig. 3b, c). When the dose of liraglutide was increased to 30 nmol kg−1 day−1, a marked reduction in food intake remained evident for the entire treatment duration (Fig. 3d). The pair-feeding necessary to match this enhanced hypophagic effect led to a significant reduction in BW in vehicle-PF animals (Fig. 3e), which was similar in magnitude to that observed in liraglutide-treated mice. Nevertheless, the 30 nmol kg−1 dose of liraglutide reduced adiposity to a greater extent compared with vehicle-PF animals (Fig. 3f). In both experiments, liraglutide and vehicle-PF mice lost significantly more lean mass than the ad libitum fed control mice; the loss in the liraglutide-treated mice with 10 nmol kg−1 and 30 nmol kg−1 being significantly larger (10 nmol kg−1 vs vehicle-PF; −1.99 ± 0.14 g vs −1.44 ± 0.17 g, p < 0.05 Tukey post hoc test) and significantly smaller (30 nmol kg−1 vs vehicle-PF; −1.74 ± 0.17 g vs −2.56 ± 0.2 g, p < 0.05), respectively, than that loss in corresponding vehicle-PF control mice.
Food intake, BW and adiposity in DIO mice chronically treated with liraglutide. Mice were given daily s.c. injections of vehicle (white squares and bars), liraglutide at a dose of 10 nmol kg−1 day−1 (a–c; black squares, black bars) or 30 nmol kg−1 day−1 (d–f; black squares, black bars), or vehicle and then pair-feeding to corresponding liraglutide-treated animals (grey squares, grey bars). Food intake in mice treated with 10 nmol kg−1 day−1 of liraglutide was transiently decreased (a) whereas BW (b; ***p < 0.001 vs vehicle; ††† p < 0.001 vs vehicle-PF, two-way ANOVA) and adiposity (c;***p < 0.001 vs vehicle; †† p < 0.01 vs vehicle-PF, one-way ANOVA with Tukey’s post hoc test) remained significantly lower compared with both vehicle-treated controls and vehicle-PF mice. s.c. injection of liraglutide at a dose of 30 nmol kg−1 day−1 reduced feeding during the entire treatment period (d; ***p < 0.001, 30 nmol kg−1 day−1 liraglutide and vehicle-PF vs vehicle, two-way ANOVA). BW was reduced in liraglutide and vehicle-PF treated mice (e; ***p < 0.001, 30 nmol kg−1 day−1 liraglutide and vehicle-PF vs vehicle, two-way ANOVA). Adiposity was also decreased in liraglutide and vehicle-PF mice and the decrease in adiposity was significantly greater in liraglutide-treated mice compared with vehicle-PF mice (f; ***p < 0.001 vs vehicle; † p < 0.05 vs vehicle-PF, one-way ANOVA with Tukey’s post hoc test). (n = 16, a–c; n = 8, d–f)
Noradrenaline-stimulated \( \overset{\cdot }{V}{\mathrm{O}}_2 \) in DIO mice chronically treated with liraglutide
To investigate the potential contribution of increased BAT metabolic capacity to the food intake-independent reduction in BW and fat mass exhibited by DIO mice treated with liraglutide, we placed the animals in sealed chambers for detailed analysis of energy balance. Locomotor activity, respiratory exchange ratio and EE were similar in all groups in both the 10 nmol kg−1 and 30 nmol kg−1 liraglutide study (data not shown). To assess changes in BAT thermogenesis, animals were adapted to 31°C and then monitored for changes in \( \overset{\cdot }{V}{\mathrm{O}}_2 \) in response to noradrenaline injection. \( \overset{\cdot }{V}{\mathrm{O}}_2 \) and AUC \( \overset{\cdot }{V}{\mathrm{O}}_2 \) in response to noradrenaline were similar in all the groups in the 10 nmol kg−1 study (Fig. 4a, b). In the study involving administration of 30 nmol kg−1 of liraglutide, both the liraglutide-treated and vehicle-PF animals had significantly lower \( \overset{\cdot }{V}{\mathrm{O}}_2 \) in response to noradrenaline injection compared with vehicle-treated mice (Fig. 4c). When \( \overset{\cdot }{V}{\mathrm{O}}_2 \) was assessed as AUC, only the data from vehicle-PF animals were significantly lower compared with vehicle-treated animals (Fig. 4d).
Noradrenaline-stimulated \( \overset{\cdot }{V}{\mathrm{O}}_2 \) in DIO mice chronically treated with liraglutide. Following 6 weeks of vehicle (white circles, white bars), liraglutide treatment at a dose of 10 nmol kg−1 day−1 (a–b; black circles, black bars), or 4 weeks with 30 nmol kg−1 day−1 (c–d; black circles, black bars), or vehicle and pair-feeding to corresponding liraglutide-treated group (grey circles, grey bars), mice were subjected to a noradrenaline s.c. injection and monitored for \( \overset{\cdot }{V}{\mathrm{O}}_2 \). No differences in \( \overset{\cdot }{V}{\mathrm{O}}_2 \) (a) or \( \Delta \overset{\cdot }{V}{\mathrm{O}}_2 \) AUC (b) were detected among the three groups in the 10 nmol kg−1 day−1 liraglutide study. In the 30 nmol kg−1 day−1 study, the 30 nmol kg−1 day−1 liraglutide-treated and vehicle-PF animals had lower \( \overset{\cdot }{V}{\mathrm{O}}_2 \) compared with vehicle-treated animals (c; *p < 0.05, **p < 0.01, two-way ANOVA with Bonferroni’s post hoc test). \( \Delta \overset{\cdot }{V}{\mathrm{O}}_2 \) AUC was significantly lower in vehicle-PF animals compared with the vehicle-treated animals (d; *p < 0.05; one-way ANOVA). (n = 7–10). NE, norepinephrine (noradrenaline)
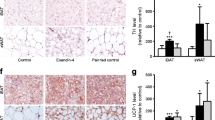
We also assessed iBAT and iWAT (Fig. 5a) and skeletal muscle (Fig. 5b, c) for changes in expression of genes involved in regulating non-shivering thermogenesis. Despite the difference in BW and fat mass among the groups we did not detect differences in the expression of genes involved in controlling BAT, iWAT or skeletal muscle activity.
Relative expression of genes involved in thermogenesis in iBAT, iWAT quadriceps and soleus of DIO mice following 6 weeks of treatment with vehicle, liraglutide (10 nmol kg−1 day−1) or vehicle-PF. Following 6 weeks of vehicle (white bars), liraglutide treatment at a dose of 10 nmol kg−1 day−1 (black bars), or vehicle and pair-feeding to the liraglutide-treated group (grey bars), gene expression was assessed in iBAT and iWAT (a) as well as in quadriceps (b) and soleus (c). Gene expression in all tissues was similar among all three groups. (n = 5–6). AU, arbitrary units
Discussion
Recent evidence demonstrating that central activation of GLP-1R signalling plays a role in the control of BAT activity raised the possibility of a contribution of BAT to the BW lowering effect following chronic administration of GLP-1 mimetics. Our data demonstrate that although direct activation of GLP-1R signalling in the brain is sufficient to increase BAT activity [3], this is not necessary for the BW lowering effects of chronic peripheral treatment with the GLP-1 mimetic liraglutide in DIO mice.
Consumption of an HFD increases EE and thermogenesis, which is referred to as DIT and is attributed to an increase in activity of BAT [22]. HFD consumption leads to an increase in brainstem preproglucagon (PPG) expression [23]. Furthermore, other studies show that the amount of adiposity in rats fed an HFD has a positive correlation with PPG gene expression [24]. This, and the recent evidence revealing a role for GLP-1R to control BAT activity [3, 4], supported the hypothesis that endogenous GLP-1R signalling contributes to increased BAT activity following HFD feeding. Here, we tested this hypothesis by comparing the \( \overset{\cdot }{V}{\mathrm{O}}_2 \) of WT and Glp1r-KO mice in response to physiological and pharmacological stimuli of BAT activity.
As a physiological stimulus, we compared the \( \overset{\cdot }{V}{\mathrm{O}}_2 \) during housing at standard temperatures of 22°C or at a thermoneutral temperature. Feldman and colleagues elegantly used this approach to unveil the role of uncoupling protein 1 (UCP1) in the protection against diet-induced obesity [7]. With this approach, we found that Glp1r-KO mice fed with an HFD for 4 weeks have lower \( \overset{\cdot }{V}{\mathrm{O}}_2 \) when compared with their WT controls when housed at 22°C but not at 31°C, which supports a role of endogenous GLP-1R in the development of DIT. Noteworthy, HFD-fed Glp1r-KO mice had a lower BW in comparison with WT controls. This resistance to diet-induced obesity is consistent with previous observations [20, 21] and its aetiology remains unknown. It may be contributed by a direct role of GLP-1R signalling in regulating adipogenesis [25] or by other compensatory signals developed in response to congenital disruption of the Glp1r gene [26]. Importantly, Glp1r-KO mice preserved the ability to adjust their energy intake in response to the changes in environmental temperature, indicating that GLP-1R signalling does not play a critical role in the homeostatic control of energy intake.
As a pharmacological stimulus of BAT activity, we performed noradrenaline injections in mice previously housed at thermoneutrality [19]. Consistent with the lower \( \overset{\cdot }{V}{\mathrm{O}}_2 \) at 22°C, HFD-fed Glp1r-KO mice exhibited a lower \( \overset{\cdot }{V}{\mathrm{O}}_2 \) compared with WT controls when challenged with noradrenaline, which strengthens the potential involvement of endogenous GLP-1R signalling in the development of BAT thermogenesis in response to high-fat feeding. Intriguingly, chow-fed, lean Glp1r-KO mice exhibited a slight but significant increase in \( \overset{\cdot }{V}{\mathrm{O}}_2 \) compared with WT controls, following noradrenaline injection. The cause of this difference in the noradrenaline-stimulated \( \overset{\cdot }{V}{\mathrm{O}}_2 \) under chow-fed conditions remains to be determined.
The impaired DIT in mice lacking endogenous GLP-1R signalling contrasts with the increased EE and increased UCP1 and beta-3 adrenergic receptor gene expression in iBAT exhibited by HFD-fed dipeptidyl peptidase 4 deficient (Dpp4 −/−) mice, a model with increased endogenous GLP-1 levels [27]. Consistently, direct CNS administration of GLP-1R ligands—including liraglutide [3, 4]—activates BAT. However, the characterisation of the control of BAT by central GLP-1R signalling was conducted in lean rodents fed standard low-fat diets undergoing relatively short periods of treatment (under 7 days) [3, 4]. These conditions differ significantly from those in which GLP-1 mimetics promote meaningful BW loss in humans. Thus, we aimed to determine the contribution of BAT thermogenesis to the weight lowering effect of chronic (4–6 weeks) pharmacological gain of GLP-1R function in a standard model of obesity such as the DIO-C57Bl6 mouse. Our data clearly demonstrate the ability of liraglutide to reduce BW and adiposity to values that exceed what could be attributable to the reduced caloric intake, likely by engaging additional physiological mechanisms that contribute to further a negative energy balance, one of which could be BAT thermogenesis. Surprisingly, we detected neither enhanced BAT activity, as measured by \( \overset{\cdot }{V}{\mathrm{O}}_2 \) in response to noradrenaline injection, nor increased expression of genes associated with increased BAT activity or ‘browning’ of WAT. We also measured thermogenic genes in quadriceps and soleus muscle and noted no changes in gene expression in response to chronic liraglutide treatment. To discard that the absence of a significant increase in BAT activity was the result of a sub-threshold dose with liraglutide, we repeated the study but this time with a higher dose (30 nmol kg−1 day−1). With the higher dose, mice experienced sustained hypophagia during the treatment period and a more pronounced BW loss. In this case, BAT thermogenesis, as measured by noradrenaline-stimulated \( \overset{\cdot }{V}{\mathrm{O}}_2 \), did not increase but was actually reduced, likely as a consequence of excessive BW loss. It could be argued that lack of effect of liraglutide on BAT activation in DIO mice was the result of intrinsically elevated DIT, but certainly, the pharmacological activation of GLP-1R signalling does not prevent its reduction associated with weight loss.
A study examining the appearance of peripherally administered liraglutide into the brain indicated that the majority of liraglutide appears in the arcuate nucleus (ARC) [28]. Lower amounts were found in the paraventricular nucleus (PVN), and little to no liraglutide appears in the ventromedial hypothalamic nucleus (VMH) or dorsomedial hypothalamic nucleus (DMH), which are the major centres regulating BAT thermogenesis [28]. Importantly, Beiroa et al gave intranuclear brain injections of liraglutide to rats and demonstrate that the VMH, not the ARC, is the site of GLP-1R-mediated activation of BAT [4]. Poor access of subcutaneously injected liraglutide to hypothalamic nuclei involved in regulating BAT activity may be contributing to the discrepancy in results of peripheral vs central administration of GLP-1R agonists on BAT activation.
Our data strongly suggest that mechanisms other than the regulation of feeding or BAT activity play a significant role in the BW lowering effect following chronic administration of GLP-1 mimetics. Another potential mechanism could be enhanced thermogenesis in skeletal muscle. UCP2 and UCP3 in skeletal muscle have been shown to play a role in adaptive thermogenesis (reviewed in [29]). Similar to upregulation of Ucp1 in BAT in response to HFD feeding, both Ucp2 and Ucp3 in quadriceps are upregulated by high-fat feeding in C57Bl6 mice, suggesting a role for skeletal muscle in mediating DIT [30]. Liraglutide-treated mice had a similar expression level of both Ucp2 and Ucp3 in skeletal muscle compared with both vehicle and vehicle-pair-fed mice, suggesting that enhanced muscle thermogenesis is not the mechanism driving the superior weight loss in the liraglutide-treated mice. The physiological and molecular basis underlying the mechanisms for enhanced weight loss remains to be determined but may be of significant importance to understand the efficacy on weight loss of therapies involving GLP-1R agonists.
In summary, our results demonstrate that although endogenous GLP-1R signalling contributes to the increase in BAT thermogenesis [3, 4], and may contribute to DIT, this is not seminal for the BW lowering effect observed with chronic peripheral treatment with the GLP-1 mimetic, liraglutide, or congenital loss of function of GLP-1R signalling. Importantly, our data demonstrate that mechanisms other than increased BAT thermogenesis play a role in the food intake-independent BW lowering effect mediated by the increase in GLP-1R signalling. Unveiling such mechanisms should provide alternative targets for the treatment of obesity.
Abbreviations
- ARC:
-
Arcuate nucleus
- BAT:
-
Brown adipose tissue
- BW:
-
Body weight
- CD:
-
Control diet
- CNS:
-
Central nervous system
- DIO:
-
Diet-induced obese
- DIT:
-
Diet-induced thermogenesis
- EE:
-
Energy expenditure
- GLP-1:
-
Glucagon-like peptide-1
- GLP-1R:
-
Glucagon-like peptide-1 receptor
- HFD:
-
High-fat diet
- iBAT:
-
Interscapular brown adipose tissue
- iWAT:
-
Inguinal white adipose tissue
- KO:
-
Knockout
- PPG:
-
Preproglucagon
- UCP:
-
Uncoupling protein
- VMH:
-
Ventromedial hypothalamic nucleus
- \( \overset{\cdot }{V}{\mathrm{O}}_2 \) :
-
Oxygen consumption
- WT:
-
Wild-type
References
Edwards KL, Stapleton M, Weis J, Irons BK (2012) An update in incretin-based therapy: a focus on glucagon-like peptide-1 receptor agonists. Diabetes Technol Ther 14:951–967
Tong J, Sandoval DA (2011) Is the GLP-1 system a viable therapeutic target for weight reduction? Rev Endocr Metab Disord 12:187–195
Lockie SH, Heppner KM, Chaudhary N et al (2012) Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes 61:2753–2762
Beiroa D, Imbernon M, Gallego R et al (2014) GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 63:3346–3358
Lockie SH, Stefanidis A, Oldfield BJ, Perez-Tilve D (2013) Brown adipose tissue thermogenesis in the resistance to and reversal of obesity: a potential new mechanism contributing to the metabolic benefits of proglucagon-derived peptides. Adipocytes 2:196–200
Lowell BB, S-Susulic V, Hamann A et al (1993) Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366:740–742
Feldmann HM, Golozoubova V, Cannon B, Nedergaard J (2009) UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab 9:203–209
Dulloo AG (2002) Biomedicine. A sympathetic defense against obesity. Science 297:780–781
Lowell BB, Spiegelman BM (2000) Towards a molecular understanding of adaptive thermogenesis. Nature 404:652–660
Bachman ES, Dhillon H, Zhang CY et al (2002) betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science 297:843–845
Rothwell NJ, Stock MJ (1979) A role for brown adipose tissue in diet-induced thermogenesis. Nature 281:31–35
Virtanen KA, Lidell ME, Orava J et al (2009) Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525
Cypess AM, Lehman S, Williams G et al (2009) Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360:1509–1517
van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM et al (2009) Cold-activated brown adipose tissue in healthy men. N Engl J Med 360:1500–1508
Celi FS (2009) Brown adipose tissue–when it pays to be inefficient. N Engl J Med 360:1553–1556
Scrocchi LA, Brown TJ, MaClusky N et al (1996) Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat Med 2:1254–1258
Tinsley FC, Taicher GZ, Heiman ML (2004) Evaluation of a quantitative magnetic resonance method for mouse whole body composition analysis. Obes Res 12:150–160
Day JW, Ottaway N, Patterson JT et al (2009) A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol 5:749–757
Cannon B, Nedergaard J (2011) Nonshivering thermogenesis and its adequate measurement in metabolic studies. J Exp Biol 214:242–253
Hansotia T, Maida A, Flock G et al (2007) Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest 117:143–152
Wilson-Perez HE, Chambers AP, Ryan KK et al (2013) Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide 1 receptor deficiency. Diabetes 62:2380–2385
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359
Knauf C, Cani PD, Ait-Belgnaoui A et al (2008) Brain glucagon-like peptide 1 signaling controls the onset of high-fat diet-induced insulin resistance and reduces energy expenditure. Endocrinology 149:4768–4777
Barrera JG, Jones KR, Herman JP, D'Alessio DA, Woods SC, Seeley RJ (2011) Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 31:3904–3913
Challa TD, Beaton N, Arnold M, Rudofsky G, Langhans W, Wolfrum C (2012) Regulation of adipocyte formation by GLP-1/GLP-1R signaling. J Biol Chem 287:6421–6430
Ali S, Lamont BJ, Charron MJ, Drucker DJ (2011) Dual elimination of the glucagon and GLP-1 receptors in mice reveals plasticity in the incretin axis. J Clin Invest 121:1917–1929
Conarello SL, Li Z, Ronan J et al (2003) Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci U S A 100:6825–6830
Secher A, Jelsing J, Baquero AF et al (2014) The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest 124:4473–4488
Schrauwen P, Hesselink M (2002) UCP2 and UCP3 in muscle controlling body metabolism. J Exp Biol 205:2275–2285
Gong DW, He Y, Reitman ML (1999) Genomic organization and regulation by dietary fat of the uncoupling protein 3 and 2 genes. Biochem Biophys Res Commun 256:27–32
Funding
This work was funded by an NIH grant (DK077975) to DPT. KMH receives funding from the Collins Medical Trust.
Duality of interest statement
RD and DPT maintain independent research collaboration with Calibrium LLC. DPT and NO are consultants for Calibrium LLC. All other authors declare that there is no duality of interest associated with their contribution to this manuscript.
Contribution statement
KMH was responsible for data collection, study design, data analysis and interpretation, and writing the manuscript; SM, JH, NO and DS contributed to conception and design, acquisition of data or analysis and interpretation of data; RD and DPT advised study conception and design of the experimental approach, and contributed to writing and critical revision of the article. All authors reviewed the manuscript and gave final approval of the version to be published. DPT is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heppner, K.M., Marks, S., Holland, J. et al. Contribution of brown adipose tissue activity to the control of energy balance by GLP-1 receptor signalling in mice. Diabetologia 58, 2124–2132 (2015). https://doi.org/10.1007/s00125-015-3651-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-015-3651-3