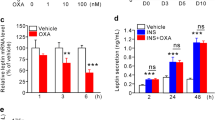
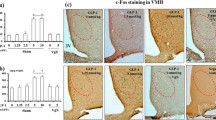
Abstract
Aims/hypothesis
Recent studies have revealed the crucial role of the central nervous system (CNS), especially the hypothalamus, in the regulation of insulin sensitivity in peripheral tissues. The aim of our current study was to investigate the possible involvement of hypothalamic prolactin receptors (PRLRs) in the regulation of hepatic insulin sensitivity.
Methods
We employed overexpression of PRLRs in mouse hypothalamus via intracerebroventricular injection of adenovirus expressing PRLR and inhibition of PRLRs via adenovirus expressing short-hairpin RNA (shRNA) specific for PRLRs in vivo. Selective hepatic vagotomy was employed to verify the important role of the vagus nerve in mediating signals from the brain to peripheral organs. In addition, a genetic insulin-resistant animal model, the db/db mouse, was used in our study to investigate the role of hypothalamic PRLRs in regulating whole-body insulin sensitivity.
Results
Overexpression of PRLRs in the hypothalamus improved hepatic insulin sensitivity in mice and inhibition of hypothalamic PRLRs had the opposite effect. In addition, we demonstrated that hypothalamic PRLR-improved insulin sensitivity was significantly attenuated by inhibiting the activity of signal transducer and activator of transcription 5 (STAT5) in the CNS and by selective hepatic vagotomy. Finally, overexpression of PRLRs significantly ameliorated insulin resistance in db/db mice.
Conclusions/interpretation
Our study identifies a novel central pathway involved in the regulation of hepatic insulin sensitivity, mediated by hypothalamic PRLR/STAT5 signalling and the vagus nerve, thus demonstrating an important role for hypothalamic PRLRs under conditions of insulin resistance.







Similar content being viewed by others
Abbreviations
- CNS:
-
Central nervous system
- ERK:
-
Extracellular signal-related kinase
- GFP:
-
Green fluorescent protein
- GTT:
-
Glucose tolerance test
- icv:
-
Intracerebroventricular
- IF:
-
Immunofluorescence
- IR:
-
Insulin receptor
- ITT:
-
Insulin tolerance test
- PRLR:
-
Prolactin receptor
- shRNA:
-
Short-hairpin RNA
- STAT5:
-
Signal transducer and activator of transcription 5
References
Chen L, Magliano DJ, Zimmet PZ (2012) The worldwide epidemiology of type 2 diabetes mellitus–present and future perspectives. Nat Rev Endocrinol 8:228–236
American Diabetes Association (2005) Diagnosis and classification of diabetes mellitus. Diabetes Care 28(Suppl 1) :S37–S42
Knight CM, Gutierrez-Juarez R, Lam TK et al (2011) Mediobasal hypothalamic SIRT1 is essential for resveratrol’s effects on insulin action in rats. Diabetes 60:2691–2700
Lam TK, Pocai A, Gutierrez-Juarez R et al (2005) Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med 11:320–327
Pocai A, Lam TK, Gutierrez-Juarez R et al (2005) Hypothalamic K(ATP) channels control hepatic glucose production. Nature 434:1026–1031
Marino JS, Xu Y, Hill JW (2011) Central insulin and leptin-mediated autonomic control of glucose homeostasis. Trends Endocrinol Metab 22:275–285
Lee JY, Muenzberg H, Gavrilova O et al (2008) Loss of cytokine-STAT5 signaling in the CNS and pituitary gland alters energy balance and leads to obesity. PLoS One 3:e1639
Goffin V, Binart N, Touraine P, Kelly PA (2002) Prolactin: the new biology of an old hormone. Annu Rev Physiol 64:47–67
Binart N, Bachelot A, Bouilly J (2010) Impact of prolactin receptor isoforms on reproduction. Trends Endocrinol Metab 21:362–368
Freemark M, Avril I, Fleenor D et al (2002) Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology 143:1378–1385
Amaral ME, Cunha DA, Anhe GF et al (2004) Participation of prolactin receptors and phosphatidylinositol 3-kinase and MAP kinase pathways in the increase in pancreatic islet mass and sensitivity to glucose during pregnancy. J Endocrinol 183:469–476
Huang C, Snider F, Cross JC (2009) Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology 150:1618–1626
Yu J, Xiao F, Zhang Q et al (2013) PRLR regulates hepatic insulin sensitivity in mice via STAT5. Diabetes 62:3103–3113
Chiu S, Wise PM (1994) Prolactin receptor mRNA localization in the hypothalamus by in situ hybridization. J Neuroendocrinol 6:191–199
Pi XJ, Grattan DR (1999) Increased expression of both short and long forms of prolactin receptor mRNA in hypothalamic nuclei of lactating rats. J Mol Endocrinol 23:13–22
Augustine RA, Kokay IC, Andrews ZB, Ladyman SR, Grattan DR (2003) Quantitation of prolactin receptor mRNA in the maternal rat brain during pregnancy and lactation. J Mol Endocrinol 31:221–232
Pi XJ, Grattan DR (1998) Differential expression of the two forms of prolactin receptor mRNA within microdissected hypothalamic nuclei of the rat. Brain Res Mol Brain Res 59:1–12
Park S, Kang S, Lee HW, Ko BS (2012) Central prolactin modulates insulin sensitivity and insulin secretion in diabetic rats. Neuroendocrinology 95:332–343
Purkayastha S, Zhang H, Zhang G et al (2011) Neural dysregulation of peripheral insulin action and blood pressure by brain endoplasmic reticulum stress. Proc Natl Acad Sci U S A 108:2939–2944
Ueda H, Ikegami H, Kawaguchi Y et al (2000) Age-dependent changes in phenotypes and candidate gene analysis in a polygenic animal model of Type II diabetes mellitus; NSY mouse. Diabetologia 43:932–938
Wang Q, Jiang L, Wang J et al (2009) Abrogation of hepatic ATP-citrate lyase protects against fatty liver and ameliorates hyperglycemia in leptin receptor-deficient mice. Hepatology 49:1166–1175
Xiao F, Huang Z, Li H et al (2011) Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes 60:746–756
Cheng Y, Zhang Q, Meng Q et al (2011) Leucine deprivation stimulates fat loss via increasing CRH expression in the hypothalamus and activating the sympathetic nervous system. Mol Endocrinol (Baltimore) 25:1624–1635
Guo F, Cavener DR (2007) The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab 5:103–114
Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799–806
Yip SH, Eguchi R, Grattan DR, Bunn SJ (2012) Prolactin signalling in the mouse hypothalamus is primarily mediated by signal transducer and activator of transcription factor 5b but not 5a. J Neuroendocrinol 24:1484–1491
Ono H, Pocai A, Wang Y et al (2008) Activation of hypothalamic S6 kinase mediates diet-induced hepatic insulin resistance in rats. J Clin Invest 118:2959–2968
Kodama H, Fujita M, Yamaguchi I (1994) Development of hyperglycaemia and insulin resistance in conscious genetically diabetic (C57BL/KsJ-db/db) mice. Diabetologia 37:739–744
Posner BI, Kelly PA, Friesen HG (1975) Prolactin receptors in rat liver: possible induction by prolactin. Science (New York) 188:57–59
Boutin JM, Jolicoeur C, Okamura H et al (1988) Cloning and expression of the rat prolactin receptor, a member of the growth hormone/prolactin receptor gene family. Cell 53:69–77
Bouilly J, Sonigo C, Auffret J, Gibori G, Binart N (2012) Prolactin signaling mechanisms in ovary. Mol Cell Endocrinol 356:80–87
Furth PA, Nakles RE, Millman S, Diaz-Cruz ES, Cabrera MC (2011) Signal transducer and activator of transcription 5 as a key signaling pathway in normal mammary gland developmental biology and breast cancer. Breast Cancer Res 13:220
White UA, Stephens JM (2010) Transcriptional factors that promote formation of white adipose tissue. Mol Cell Endocrinol 318:10–14
Cui Y, Hosui A, Sun R et al (2007) Loss of signal transducer and activator of transcription 5 leads to hepatosteatosis and impaired liver regeneration. Hepatology 46:504–513
Filippi BM, Yang CS, Tang C, Lam TK (2012) Insulin activates Erk1/2 signaling in the dorsal vagal complex to inhibit glucose production. Cell Metab 16:500–510
Pocai A, Obici S, Schwartz GJ, Rossetti L (2005) A brain-liver circuit regulates glucose homeostasis. Cell Metab 1:53–61
van den Hoek AM, Voshol PJ, Karnekamp BN et al (2004) Intracerebroventricular neuropeptide Y infusion precludes inhibition of glucose and VLDL production by insulin. Diabetes 53:2529–2534
Zhang Q, Yu J, Liu B et al (2013) Central activating transcription factor 4 (ATF4) regulates hepatic insulin resistance in mice via S6K1 signaling and the vagus nerve. Diabetes 62:2230–2239
Byrnes AP, Rusby JE, Wood MJA, Charlton HM (1995) Adenovirus gene-transfer causes inflammation in the brain. Neuroscience 66:1015–1024
Purkayastha S, Cai D (2013) Neuroinflammatory basis of metabolic syndrome. Mol Metab 2:356–363
Moderscheim TAE, Gorba T, Pathipati P et al (2007) Prolactin is involved in glial responses following a focal injury to the juvenile rat brain. Neuroscience 145:963–973
Obici S, Zhang BB, Karkanias G, Rossetti L (2002) Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8:1376–1382
Shingo T, Gregg C, Enwere E et al (2003) Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 299:117–120
Ladyman SR, Augustine RA, Grattan DR (2010) Hormone interactions regulating energy balance during pregnancy. J Neuroendocrinol 22:805–817
Melo AM, Benatti RO, Ignacio-Souza LM et al (2014) Hypothalamic endoplasmic reticulum stress and insulin resistance in offspring of mice dams fed high-fat diet during pregnancy and lactation. Metab Clin Exp 63:682–692
Acknowledgements
We thank W. Qiao (Institute of Neuroscience, Shanghai, China) for assistance with materials.
Funding
This work was supported by grants from the National Natural Science Foundation (81130076, 31271269, 81100615, 30890043, 81390350 and 81300659), Ministry of Science and Technology of China (973 Program 2010CB912502), International S&T Cooperation Program of China (Singapore 2014DFG32470), China National Funds for Distinguished Young Scientists (81325005), Basic Research Project of Shanghai Science and Technology Commission (13JC1409000), Key Program of Shanghai Scientific and Technological Innovation Action Plan (10JC1416900), the Knowledge Innovation Program of CAS (KSCX2-EW-R-09) and Chinese Academy of Sciences-funded project (2011KIP307). FG was also supported by the One Hundred Talents Program of the Chinese Academy of Sciences. FX was supported by the China Postdoctoral Science Foundation funded project (2012M520950 and 2013T60473) and a Chinese Academy of Sciences-funded project (2013KIP310).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
All authors contributed to the conception and design or the analysis and interpretation of data, and to drafting the article or revising it critically for intellectual content. All authors gave final approval of the version to be published. FG is responsible for the integrity of the work as a whole.
Author information
Authors and Affiliations
Corresponding author
Additional information
Fei Xiao, Tingting Xia and Ziquan Lv contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Methods
(PDF 152 kb)
ESM Fig. 1
(PDF 267 kb)
Rights and permissions
About this article
Cite this article
Xiao, F., Xia, T., Lv, Z. et al. Central prolactin receptors (PRLRs) regulate hepatic insulin sensitivity in mice via signal transducer and activator of transcription 5 (STAT5) and the vagus nerve. Diabetologia 57, 2136–2144 (2014). https://doi.org/10.1007/s00125-014-3336-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3336-3