Abstract
Aims/hypothesis
Chronic inflammatory processes have been increasingly shown to be involved in the pathogenesis of diabetes and diabetic nephropathy. Recently, we demonstrated that a lectin-like domain of thrombomodulin (THBD), which is known as THBD domain 1 (THBDD1) and which acts independently of protein C activation, neutralised an inflammatory response in a mouse model of sepsis. Here, therapeutic effects of gene therapy with adeno-associated virus (AAV)-carried THBDD1 (AAV-THBDD1) were tested in a mouse model of type 2 diabetic nephropathy.
Methods
To assess the therapeutic potential of THBDD1 and the mechanisms involved, we delivered AAV-THBDD1 (1011 genome copies) into db/db mice and tested the effects of recombinant THBDD1 on conditionally immortalised podocytes.
Results
A single dose of AAV-THBDD1 improved albuminuria, renal interstitial inflammation and glomerular sclerosis, as well as renal function in db/db mice. These effects were closely associated with: (1) inhibited activation of the nuclear factor κB (NF-κB) pathway and the NACHT, LRR and PYD domains-containing protein 3 (NLRP3) inflammasome; (2) promotion of nuclear factor (erythroid-derived 2)-like 2 (NRF2) nuclear translocation; and (3) suppression of mitochondria-derived apoptosis in the kidney of treated mice.
Conclusions/interpretation
AAV-THBDD1 gene therapy resulted in improvements in a model of diabetic nephropathy by suppressing the NF-κB–NLRP3 inflammasome-mediated inflammatory process, enhancing the NRF2 antioxidant pathway and inhibiting apoptosis in the kidney.
Similar content being viewed by others
Introduction
Type 2 diabetes is a leading cause of end-stage kidney disease due to diabetic nephropathy (DN) [1, 2], which is characterised by progressive albuminuria and a gradual decline in GFR, in association with progressive renal fibrosis or sclerosis, leading to ultimate chronic renal failure [3]. Although many therapeutic remedies focusing on hyperglycaemia and high blood pressure have been used, many patients still suffer progressive and severe renal injury [4], making the investigation of other pathogenic pathways and relevant therapeutic strategies worthwhile. Importantly, immune-mediated inflammatory processes [5], apoptosis [6, 7] and reactive oxygen species (ROS) [5, 8] in the kidney are involved in the developmental and progressive stages of DN.
Thrombomodulin (THBD), a component of the anticoagulant pathway, binds to thrombin on the endothelial cell surface and catalyses activation of protein C, thus protecting cells from thrombus formation [9, 10]. THBD consists of five domains: a lectin-like domain of THBD known as THBD domain 1 (THBDD1), followed by a domain containing six epidermal growth factor repeats, a serine- and threonine-rich domain, a transmembrane domain and a cytoplasmic domain. Previously, recombinant THBD has been shown to improve certain types of acute renal damage, such as thrombotic glomerulonephritis [11] and acute ischaemic renal injury [12]. However, studies by our team [13] and others [14] have shown that THBD also has a protein C- and thrombin-independent physiological function. In agreement with this notion, we recently demonstrated that recombinant THBDD1 (rTHBDD1) can inhibit an inflammatory response by neutralising lipopolysaccharide through binding to Lewis Y antigen [15] and has anti-angiogenesis effects on cultured HUVECS [16]. We also demonstrated that cardiomyocytes are protected from apoptosis by THBD treatment as a result of its effect on the balance of B cell leukaemia/lymphoma 2 (BCL-2) and BCL-2-associated X protein (BAX) levels [17]. The anti-apoptotic effect of THBD has also been confirmed in studies using endothelial cells and neurons [12].
In the present study, we delivered adeno-associated virus (AAV)-carried THBDD1 (AAV-THBDD1) into db/db mice, which had already developed hyperglycaemia. We found that these animals were protected from renal inflammation and fibrosis or sclerosis through the inhibition of inflammation, activation of the nuclear factor (erythroid-derived 2)-like 2 (NRF2) antioxidant pathway and prevention of apoptosis.
Methods
Preparation of rTHBDD1 protein and AAV-THBDD1
The production of rTHBDD1 and AAV serotype 2/8 vectors encoding human THBDD1 (amino acid 1-155) (AAV-THBDD1) has been described previously [16].
Animal experiments
Male C57BLKs/J db/db mice and their age-matched, normal, wild-type (WT) db/m littermates were purchased from Jackson Laboratories (Bar Harbor, ME, USA). The mice were classified into three groups (n = 12 each): (1) AAV empty vector-treated db/db mice (vector-alone control db/db); (2) AAV-THBDD1-treated mice; and (3) WT mice as normal control. We injected the mice intravenously with a single dose of AAV or AAV-THBDD1. This dose (1011 genome copies) was chosen according to our previous study because it caused no detectable pathological effects in the mice [16] by the age of 32 weeks (week 0 after the AAV or AAV-THBDD1 injection), i.e. at a time point when hyperglycaemia and hyperglycosuria had already developed and just before the onset of proteinuria. The db/db mice injected with the empty AAV vector served as disease controls. All the mice were killed 18 weeks after the AAV or AAV-THBDD1 injections and samples were collected for individual analyses as described previously [7], with slight modifications. All animal experiments were performed after approval by the Institutional Animal Care and Use Committee of The National Defense Medical Center, Taiwan, and are consistent with the NIH Guide for the Care and Use of Laboratory Animals.
Clinical and histopathological evaluation
The collection and assay of blood and urine samples were done as described previously [7]. For histopathological evaluation, mesangial expansion (increased matrix), and sclerosis and hyalinosis in the glomerulus, as well as renal interstitial inflammation were evaluated by light microscopy and scored as described previously [18].
Immunofluorescence
Frozen sections of the kidney were subjected to immunofluorescence staining with goat anti-mouse IgG, IgM and C3 (Sigma, St Louis, MO, USA). The source of FITC-conjugated, labelled IgG, IgM and C3 has been described previously [19].
Confocal microscopy of podocyte injury in vivo
Frozen sections were stained with guinea pig anti-nephrin antibody (Acris Antibodies, Herford, Germany) and rabbit antibody against vascular endothelial growth factor (VEGF) (Santa Cruz Biotechnology, Dallas, TX, USA), followed by Alexa Fluor 488-labelled rabbit anti-guinea pig IgG antibody (Invitrogen, Grand Island, NY, USA) or phycoerythrin-labelled goat anti-rabbit IgG antibody (Jackson ImmunoResearch, West Grove, PA, USA) as described previously [7].
Western blot analysis
Rabbit antibodies against mouse NACHT, LRR and PYD domains-containing protein 3 (NLRP3) (Zymed, South San Francisco, CA, USA), caspase-3, caspase-8 or caspase-9 (Cell Signaling Technology, Frankfurt am Main, Germany), and BCL-2 or BAX, as well as goat antibodies against mouse NRF2 or β-actin (Santa Cruz Biotechnology), were used as described previously [18].
ELISA
Serum levels of IL-1β and monocyte chemoattractant protein 1 (MCP-1) (eBioscience, San Diego, CA, USA), and renal haem oxygenase-1 (HO-1) levels (Enzo Life Sciences, Farmingdale, NY, USA) were measured using commercial ELISA kits according to the manufacturers’ instructions.
Enzyme activity assay
Renal glutathione peroxidase (GPx) activity (Cayman, Ann Arbor, MI, USA), and caspase-1, caspase-3 and caspase-9 activity (R&D, Minneapolis, MN, USA) were measured using commercial ELISA kits according to the manufacturers’ instructions. Renal phosphorylated nuclear factor κB (NF-κB) p65 activity was measured using an ELISA assay kit (TransAM; Active Motif, Carlsbad, CA, USA) according to the manufacturer’s instructions and a multi-mode ELISA plate reader (Bio-Tek, Winooski, VT, USA).
Measurement of ROS production
ROS levels in serum and renal tissue were measured as described previously [18]. ROS activity was expressed as relative luminescence units (RLU) per 15 min per mg of organ dry weight, i.e. RLU/mg or RLU/ml.
In vitro analyses of podocytes
Conditionally immortalised mouse podocytes were obtained from S. J. Shankland (Department of Medicine, University of Washington, Seattle, WA, USA) and differentiation induced by culturing the cells for 13 to 15 days at 37°C in the absence of IFN-γ (Roche, Nutley, NJ, USA), as described previously [20].
Apoptosis
Podocytes (5 × 104) were incubated for 1 h with or without rTHBDD1 (5, 17 or 50 μg/ml), and then incubated for 12 or 24 h with medium containing normal glucose (NG) (5 mmol/l d-glucose) as a control or high glucose (HG) (30 mmol/l d-glucose). The cells were then collected and analysed for (1) caspase-3 protein abundance by western blot analysis and (2) apoptosis by flow cytometry, as described previously [21].
Protein content of high-mobility group box 1 and MCP-1 in podocytes
Podocytes (1 × 104) were incubated with or without rTHBDD1 (50 μg/ml), and with NG (5 mmol/l d-glucose), HG (30 mmol/l d-glucose) or NG (5 mmol/l d-glucose) + mannitol (25 mmol/l; used as an osmolality control). For immunofluorescence, the cells were incubated with rabbit anti-high-mobility group box 1 (HMGB1) (Abcam, Cambridge, UK) antibody, followed by FITC-labelled goat anti-rabbit IgG antibody (Jackson ImmunoResearch) and counterstaining with DAPI (Sigma). Cells were then measured by fluorescence microscopy at a magnification of ×400. The levels of MCP-1 in the culture medium were measured by ELISA (eBioscience) according to the manufacturer’s instructions.
Statistical analysis
Values are presented as the mean ± SEM. Comparisons of serum levels of THBD and sugar, and urine levels of albumin and glucose between groups were made with one-way ANOVA. Comparison between two groups was performed using Student’s t test. A value of p < 0.05 was considered statistically significant.
Results
Expression of Thbdd1 mRNA in liver tissue and THBDD1 protein in serum
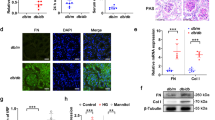
mRNA levels of Thbdd1 were significantly elevated in liver tissue of AAV-THBDD1-treated db/db mice (Electronic supplementary material [ESM] Fig. 1a, methods are provided in the ESM Methods) compared with vector-alone control db/db mice (AAV-injected only) and control db/m (WT) mice at week 18 when the animals were killed. At weeks 1 and 2, AAV-THBDD1-treated db/db mice had increased serum THBDD1 levels compared with those of vector-alone control db/db mice and WT mice, respectively, with levels peaking in week 4 after AAV-THBDD1 administration and remaining persistently high until week 18 (p < 0.005) (ESM Fig. 1b, methods are provided in the ESM Methods). These findings indicate that systemic THBDD1 expression was present in the circulation of animals that received the gene therapy.
THBDD1 improves albuminuria and renal function, but has no effect on the treatment of hyperglycaemia or hyperglycosuria
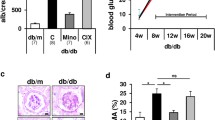
As shown in Fig. 1a, vector-alone control db/db mice first showed a significantly higher urinary albumin:creatinine ratio than WT mice at week 3 after injection, with this ratio continuing to increase until animals were killed. This effect was markedly decreased in AAV-THBDD1-treated db/db mice (p < 0.01). At week 18 after injection, AAV-THBDD1-treated db/db mice had significantly reduced serum levels of blood urea nitrogen (BUN) (Fig. 1b) and creatinine (Fig. 1c) (p < 0.05 for both) compared with vector-alone control db/db mice. However, there was no significant difference in serum or urinary glucose levels between AAV-THBDD1-treated and vector-alone control db/db mice (serum glucose AAV-THBDD1-treated db/db 32 ± 1.7 mmol/l, vector-alone control db/db 33.8 ± 1.9 mmol/l; urinary glucose AAV-THBDD1-treated db/db 5+ (>111.1 mmol/l) vs vector-alone control db/db 5+ (>111.1 mmol/l), although both groups had significantly higher serum and urinary glucose levels than WT mice (serum glucose 6.5 ± 0.7 mmol/l, urinary glucose 0+; p < 0.005 for both). There was no difference in physical activity between AAV-THBDD1-treated and vector-alone control db/db mice, although both groups of mice were obviously overweight compared with WT mice. No evidence of spontaneous internal bleeding was seen in AAV-THBDD1-treated db/db mice.
Proteinuria, renal function and renal pathology. (a) Time course of appearance of proteinuria. Black circles, WT (db/m); white circles, vector-alone control db/db; black triangles, AAV-THBDD1-treated db/db; Cr, creatinine. The arrow indicates the time of injection of AAV-THBDD1 or vector. (b) Serum BUN levels at week 18 after injection. (c) Serum creatinine levels at week 18 after injection. (d–f) Haematoxylin and eosin (HE) staining. Arrows indicate zones of fibrosis: original magnification ×400. (g) Scoring of glomerular sclerosis. ND, not detectable. *p < 0.05, **p < 0.01 and † p < 0.005
THBDD1 improves renal pathological lesions
Light microscopy revealed that vector-alone control db/db mice had diffuse mesangial expansion (increased matrix), and scattered nodular sclerosis and hyalinosis in the glomeruli (Fig. 1d–g), associated with disperse mononuclear leucocyte infiltration of the renal interstitium, although the renal tubules were mainly intact. However, these changes were substantially improved in AAV-THBDD1-injected db/db mice. As shown by immunohistochemistry, levels of collagen IV (ESM Fig. 2, methods are provided in the ESM Methods) were significantly higher in vector-alone control db/db mice than in WT mice and substantially lower in AAV-THBDD1-treated db/db mice (p < 0.05). Immunofluorescence showed that IgG, IgM and C3 were diffusely present in the glomerulus of vector-alone control and AAV-THBDD1-treated db/db mice compared with the negative staining seen in the kidney of WT mice. There was no significant difference in IgG, IgM or C3 deposits between vector-alone control and AAV-THBDD1-treated db/db mice (data not shown).
THBDD1 decreases serum and renal ROS levels, and increases renal NRF2 nuclear translocation
Vector-alone control db/db mice had significantly higher serum (Fig. 2a) and renal (Fig. 2b) levels of superoxide anions than WT mice, this effect being significantly reduced in AAV-THBDD1-treated db/db mice (p < 0.005 for both). Consistent with this, renal AAV-THBDD1-treated db/db mice had fewer dihydroethidium-positive cells than vector-alone control db/db mice (ESM Fig. 3, methods are provided in the ESM Methods). We measured protein levels of NRF2 in the nucleus (active NRF2), and GPx activity and HO-1 levels (both involved in the NRF2 pathway) in renal tissues. Compared with vector-alone control db/db mice, AAV-THBDD1-treated db/db mice had significantly higher nuclear NRF2 protein levels (Fig. 2c, d). Vector-alone control db/db mice also had much lower GPx activity than WT mice, an effect prevented by AAV-THBDD1 treatment (Fig. 2e), while HO-1 content was significantly higher in AAV-THBDD1-treated db/db mice than in vector-alone control db/db or WT mice (Fig. 2f).
ROS levels and NRF2 antioxidant signalling pathway in the kidney. (a) ROS levels in serum or (b) renal tissues. (c) Representative western blots for nuclear NRF2 with β-actin as the loading control and (d) semi-quantitative analysis of the NRF2:β-actin ratio from the western blot data. (e) Renal cytosolic GPx activity and (f) HO-1 levels. *p < 0.05, **p < 0.01 and † p < 0.005
THBDD1 inhibits NF-κB activation, MCP-1 expression and infiltration of macrophages in the kidney
As shown in Fig. 3a, vector-alone control db/db mice had significantly greater renal nuclear pNF-κB p65 activity than WT mice, this effect being completely suppressed in AAV-THBDD1-treated db/db mice (p < 0.005). Vector-alone control db/db mice also had significantly higher MCP-1 levels in serum than WT mice as assessed by ELISA (Fig. 3b). This effect was markedly inhibited in AAV-THBDD1-treated db/db mice. Furthermore, significant renal interstitial infiltration of macrophages (F4/80-positive) was seen in vector-alone control db/db mice compared with WT mice and was also markedly inhibited in AAV-THBDD1-treated db/db mice (Fig. 3c–e). In addition to the above, we also performed an in vitro experiment. As shown in Fig. 3f, rTHBDD1 significantly suppressed protein levels of MCP-1 in HG-treated podocytes at early (12 h) and late (24 h) time points. There was no significant difference in MCP-1 protein levels between osmolality control (NG + mannitol), osmolality control + rTHBDD1 or NG + rTHBDD1 compared with NG alone conditions at 12 and 24 h, respectively.
Renal NF-κB activation and related cytokines. (a) Renal pNF-κB p65 activity and (b) serum MCP-1 levels measured by ELISA. (c) Immunohistochemistry study of renal F4/80 (macrophage) expression. Arrows indicate F4/80-positive cells; original magnification ×400. (d) Percentage of positive staining for F4/80 in the glomerulus and (e) tubulointerstitial compartment. gcs (d), glomerular cross section. (f) MCP-1 in podocytes cultured in medium, measured by ELISA. Glu., glucose; Man., mannitol. *p < 0.05, **p < 0.01 and † p < 0.005
THBDD1 inhibits NLRP3 inflammasome activation and HMGB1 production
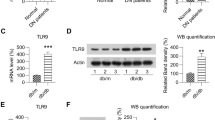
Vector-alone control db/db mice had markedly higher serum levels of IL-1β (Fig. 4a) and IL-18 (Fig. 4b) than WT mice; levels of both were significantly decreased in AAV-THBDD1-treated db/db mice. Renal NLRP3 levels were significantly increased in vector-alone control db/db mice compared with WT mice, while AAV-THBDD1 treatment resulted in a significant reduction of NLRP3 levels (Fig. 4c, d). AAV-THBDD1-treated mice also had significantly decreased renal caspase-1 activity compared with vector-alone control db/db mice (Fig. 4e). Immunohistochemistry studies showed that renal IL-1β expression in vector-alone control db/db mice was significantly higher than in WT mice and that this effect was markedly inhibited in AAV-THBDD1-treated db/db mice (ESM Fig. 4, methods are provided in the ESM Methods).
NLRP3 inflammasome activation. (a) Serum levels of IL-1β or (b) IL-18 measured by ELISA. (c) Representative western blots for renal cytosolic NLRP3 with β-actin as loading control and (d) semi-quantitative analysis of the NLRP3: β-actin ratio from the western blot data. (e) Renal cytosolic caspase-1 activity by ELISA. (f) Immunofluorescence staining of HMGB1 in cultured podocytes incubated in medium as indicated; original magnification ×400. Arrows indicate positive staining. **p < 0.01 and † p < 0.005
Immunofluorescence studies showed that cytosolic HMGB1 protein content was significantly increased in saline-treated podocytes cultured in HG medium compared with NG medium and that this HG effect was significantly inhibited in cells treated with rTHBDD1 (Fig. 4f). There was no significant difference in cytosolic HMGB1 protein content in osmolality control (NG + 25 mmol/l mannitol) compared with NG conditions.
THBDD1 prevents loss of nephrin and inhibits VEGF expression in the glomerulus
As shown in Fig. 5, vector-alone control db/db mice had less nephrin in the glomeruli than WT mice (22.7 ± 0.2% of the total glomerular area positive for nephrin, compared with 39.9 ± 1.4% in WT; p < 0.05), while AAV-THBDD1-treated db/db mice had significantly increased nephrin content in the glomerulus (32.8 ± 1.5%) compared with vector-alone control db/db mice (p < 0.05). Vector-alone control db/db mice also had significantly increased glomerular VEGF content in podocytes (19.7 ± 0.5% of the total glomerular area positive for VEGF) compared with WT mice (3.2 ± 1.8%) (p < 0.01), whereas AAV-THBDD1-treated db/db mice had only faint VEGF staining (5.6 ± 0.2%; p < 0.01 compared with vector-alone control db/db mice).
THBDD1 inhibits apoptosis in the glomerulus and renal tubule
As shown by TUNEL staining, vector-alone control db/db mice had greatly increased renal apoptosis mainly in podocytes compared with WT mice, an effect that was significantly inhibited (p < 0.05) in AAV-THBDD1-treated db/db animals (ESM Fig. 5, methods are provided in the ESM Methods). Examination of renal levels of activated caspase-8 and caspase-9 revealed no evidence for caspase-8 activation in vector-alone control db/db or AAV-THBDD1-treated db/db mice compared with WT mice (data not shown), but levels of the mature form (p35 fragment) of caspase-9 were greatly increased in vector-alone control db/db mice compared with WT mice, this effect being suppressed in AAV-THBDD1-treated db/db mice (p < 0.005) (Fig. 6a, b). In addition, the mature form (p17 fragments) of renal caspase-3 was increased in vector-alone control db/db mice, but this effect was greatly suppressed in AAV-THBDD1-treated db/db mice (p < 0.005) (Fig. 6a, c). We also measured renal levels of BAX and BCL-2, finding that the ratio was increased in vector-alone control db/db mice compared with WT mice, an effect that was significantly decreased (p < 0.01) in AAV-THBDD1-treated db/db mice (Fig. 6a, d). AAV-THBDD1-treated mice also had significantly decreased renal caspase-9 and caspase-3 activity compared with vector-alone control db/db mice (Fig. 6e, f). These findings suggest that the THBDD1-induced reduction in renal apoptosis is due to suppression of the intrinsic pathway of apoptosis and reduction of the BAX: BCL-2 ratio.
Western blot analysis for apoptosis. (a) Representative western blots for renal caspase-9 p35, caspase-3 p17, BCL-2 and BAX, with semi-quantitative analysis of the (b) active caspase-9 p35:β-actin, (c) caspase-3 p17:β-actin and (d) BAX:BCL-2 ratios from the western blot data. (e) Renal cytosolic caspase-9 and (f) caspase-3 activity by ELISA. Cultured podocytes were incubated in NG medium (5 mmol/l), HG medium (30 mmol/l) or HG plus rTHBDD1 (5, 17 or 50 μg/ml). *p < 0.05, **p < 0.01 and † p < 0.005
We examined the time course and dose-dependent effects of rTHBDD1 treatment on cultured podocytes, which have been shown to undergo apoptosis in HG medium [22]. As shown in Fig. 7a, b, at 24 h the percentage of annexin V- and propidium iodide (PI)-positive cells was significantly increased in HG-treated compared with NG-treated podocytes, this HG effect being significantly inhibited in cells treated with rTHBDD1 at 50 μg/ml (p < 0.005). This finding was confirmed by the observation that the increased levels of the mature form (p17) of caspase-3 seen in HG-treated podocytes were completely suppressed in rTHBDD1-treated podocytes at 24 h (p < 0.005) (Fig. 7c, d). No difference in the percentage of apoptotic cells was observed between NG-treated, HG-treated and HG + rTHBDD1-treated podocytes at 12 h (data not shown). In addition, only 50 μg/ml of rTHBDD1 was capable of inhibiting apoptosis in HG-treated podocytes, as demonstrated by flow cytometry and western blot analysis.
Flow cytometry for apoptosis. (a) Flow cytometry apoptosis assay of annexin V and PI positivity (encircled area by continuous lines indicates apoptotic population) with (b) annexin V- and PI-positive findings in per cent. (c) Representative western blots for caspase-3 p17 in cultured podocytes and (d) semi-quantitative analysis of the activated caspase-3 p17:β-actin ratio. Cultured podocytes were incubated in NG (5 mmol/l), HG (30 mmol/l) or HG plus rTHBDD1 (5, 17 or 50 μg/ml) medium. † p < 0.005
Discussion
In a recent study, we showed that administration of AAV serotype 2/8 carrying THBDD1, which was also used in the present study, can cause hepatic expression of THBDD1 and release of the protein into blood, thereby inhibiting angiogenesis in rats [16]. Other authors [23] have shown that recombinant AAV serotype 2/8 vectors are a promising tool for long-term tissue protein expression in vivo. Consistent with this, we showed significantly increased expression of Thbdd1 mRNA in liver tissue of AAV-THBDD1-treated db/db mice, with the THBDD1 protein released into blood exerting a renoprotective effect on renal lesions of the treated mice.
Our data provide the first demonstration, to our knowledge, that AAV-THBDD1 gene therapy exerts its beneficial effects in db/db mice as a result of anti-inflammatory effects caused by suppressing the activation of NF-κB and the NLRP3 inflammasome, enhancing the NRF2 antioxidant pathway and inhibiting mitochondria-derived apoptosis in the kidney.
First, AAV-THBDD1 administration resulted in inhibition of NF-κB activation, F4/80-positive macrophage infiltration and NLRP3 inflammasome activation in the kidney compared with vector-alone control db/db mice. These results show that, although intact THBD can activate protein C and activated protein C (APC) has anti-inflammatory and anticoagulation properties, the effect of THBDD1 in AAV-THBDD1-treated db/db mice was probably independent of APC. This finding is confirmed by our recent observation, in a mouse model of sepsis, that THBDD1 has APC-independent anti-inflammatory activity in vivo and inhibits neutrophil infiltration into the kidney [15]. Collectively, ours and several lines of research have suggested possible mechanisms for the direct anti-inflammatory effects of THBDD1, including: (1) interference with the binding of lipopolysaccharide to its receptor and inhibition of inflammatory mediator production by macrophages [15]; (2) suppression of adhesion molecule expression by neutrophils acting via the NF-κB and MAPK pathways [24]; (3) prevention of leucocyte activation by sequestration of HMGB1 protein [25]; and (4) interference with complement activation (C3a and C5a) via the classical and lectin pathways [26]. In addition, our previous study showed that anti-inflammatory effects can significantly ameliorate DN in db/db mice [7] by blocking the NF-κB-mediated inflammatory pathway. We did not identify the sequence of events in vivo, which include reduced inflammation, reduced oxidative stress, increased antioxidant capacity and reduced apoptosis as the possible basis for the protective effects of THBDD1. Nevertheless, reduced inflammation might well be the key mechanism involved. Very recently, Wang et al also demonstrated that THBD controls DN through its lectin-like domain by regulating complement independently of APC [27]. In this regard, our data show that there is no significant difference in C3 deposits between vector-alone control db/db mice and AAV-THBDD1-treated db/db mice. It is likely that THBDD1 achieved its preventive effects in the model of DN without improving glomerular localisation of C3 in the treated mice.
NLRP3 inflammasome has been shown to play a role in the pathogenesis of type 2 diabetes and DN in humans [28, 29], and in a model of type 2 diabetes [30]. Our data suggest that modulation of the NLRP3 inflammasome pathway by AAV-THBDD1 administration helps prevent renal inflammation and fibrosis in our mouse model of DN. Additional evidence supporting the anti-inflammatory effects of THBDD1 comes from the decreased HMGB1 protein levels in HG medium-cultured podocytes that were treated with rTHBDD1. This observation is consistent with findings that HMGB1 and receptors for AGE are blocked by THBDD1, thereby inhibiting HMGB1-mediated inflammatory reactions [25]. Increased serum levels of THBD domains have been observed in patients with diabetes [31–33] and some specific forms of THBD domains were differentially expressed in patients with DN and non-diabetic nephritis [33]; however, the role of THBD in these renal conditions was not determined. In the present study, the beneficial effects of THBDD1 on the db/db mice treated suggest that THBD may be protective to diabetes-associated renal damage, although further investigation on this point is warranted.
Excessive ROS production has been shown to increase the expression of extracellular matrix proteins involved in the development of renal fibrosis [18], and to play a major pathogenic role in the development and complications of diabetes [8]. Blocking of cellular signalling pathways leading to ROS generation has been shown to blunt DN [34]. Interestingly, NRF2 has been shown to exert its beneficial effects on DN [29] and other renal conditions [18] by countering oxidative stress. Moreover, the data [18, 27] on renal HO-1 in AAV-THBDD1-treated animals are not consistent with the entirety of the other data in our study, where renal HO-1 levels were greater than in WT mice and vector-alone control db/db mice. This issue needs to be addressed. It is established that expression levels or the activity of HO-1 and GPx are involved in the elimination or inactivation of ROS [35], and that this effect is mediated by activation of NRF2 [36]. Although normal tissues express extremely low basal levels of HO-1 protein [37, 38], diabetic rats receiving antioxidant treatment have significantly increased HO-1 protein levels compared with normal controls [39]. Together, our data suggest that the expression of HO-1 protein may be a key factor in preventing mice from developing renal lesions in DN.
Podocyte loss has been implicated in the progression of DN [6, 7], and this has been confirmed in mouse models of type 2 diabetes, which show apoptosis in various compartments of the kidney [6, 8]. Our in vitro data showed that rTHBDD1 had a significant inhibitory effect on podocyte apoptosis induced in a HG environment and that AAV-THBDD1 gene delivery inhibited apoptosis in renal tissues via a caspase-9 intrinsic pathway. However, the concentration of THBDD1 used in vitro was relatively high, and the effects demonstrated could therefore have been due to a charge effect alone of the THBDD1 used. To address this issue, we performed an experiment with HG-treated podocytes, in which BSA, which bears approximately the same net charge as rTHBDD1 (its net charge is 4.9), was added to the cell culture in a dose-dependent and time-dependent manner. No detectable inhibitory effect on apoptosis of the cells was observed by flow cytometry with annexin V- and PI-positive staining (data not shown).
THBD activates protein C and APC has an anticoagulant effect, with the associated risk of bleeding [40]. No evidence of systemic side effects, including bleeding, was seen in AAV-THBDD1-treated db/db mice, suggesting that THBDD1 is relatively safe for use in vivo for at least 18 weeks at the dose used in this study.
In the present study, albuminuria and histological changes were approximately halved in treated mice, yet most other endpoints addressing intermediates appeared to be normalised. The in vivo microenvironment is very complicated and changes in each variable may vary according to different analytical techniques. The question of whether this effect, or other causes, can explain the observed discrepancy requires further investigation.
Abbreviations
- AAV:
-
Adeno-associated virus
- APC:
-
Activated protein C
- BAX:
-
BCL-2-associated X protein
- BCL-2:
-
B cell leukaemia/lymphoma 2
- BUN:
-
Blood urea nitrogen
- DN:
-
Diabetic nephropathy
- GPx:
-
Glutathione peroxidase
- HG:
-
High glucose
- HMGB1:
-
High-mobility group box 1
- HO-1:
-
Haem oxygenase-1
- MCP-1:
-
Monocyte chemoattractant protein 1
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NG:
-
Normal glucose
- NLRP3:
-
NACHT, LRR and PYD domains-containing protein 3
- NRF2:
-
Nuclear factor (erythroid-derived 2)-like 2
- PI:
-
Propidium iodide
- RLU:
-
Relative luminescence units
- ROS:
-
Reactive oxygen species
- rTHBDD1 :
-
Recombinant THBDD1
- THBD:
-
Thrombomodulin
- THBDD1:
-
THBD domain 1
- VEGF:
-
Vascular endothelial growth factor
- WT:
-
Wild-type
References
Booth GL, Kapral MK, Fung K, Tu JV (2006) Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet 368:29–36
Wolf G, Ritz E (2003) Diabetic nephropathy in type 2 diabetes prevention and patient management. J Am Soc Nephrol 14:1396–1405
Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH (2004) Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int 65:116–128
Stolar M (2010) Glycemic control and complications in type 2 diabetes mellitus. Am J Med 123:S3–S11
Elmarakby AA, Sullivan JC (2012) Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc Ther 30:49–59
Isermann B, Vinnikov IA, Madhusudhan T et al (2007) Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med 13:1349–1358
Ka SM, Yeh YC, Huang XR et al (2012) Kidney-targeting Smad7 gene transfer inhibits renal TGF-beta/MAD homologue (SMAD) and nuclear factor kappaB (NF-kappaB) signalling pathways, and improves diabetic nephropathy in mice. Diabetologia 55:509–519
Brezniceanu ML, Liu F, Wei CC et al (2008) Attenuation of interstitial fibrosis and tubular apoptosis in db/db transgenic mice overexpressing catalase in renal proximal tubular cells. Diabetes 57:451–459
Morser J (2012) Thrombomodulin links coagulation to inflammation and immunity. Curr Drug Targets 13:421–431
Ohsawa I, Ohi H, Fujita T, Kanmatsuse K (1996) Elevation of plasma thrombomodulin level in primary glomerulonephritis with heavy proteinuria. Nihon Jinzo Gakkai Shi 38:300–304
Ikeguchi H, Maruyama S, Morita Y et al (2002) Effects of human soluble thrombomodulin on experimental glomerulonephritis. Kidney Int 61:490–501
Ozaki T, Anas C, Maruyama S et al (2008) Intrarenal administration of recombinant human soluble thrombomodulin ameliorates ischaemic acute renal failure. Nephrol Dial Transplant Off Publ Eur Dial Transplant Assoc Eur Ren Assoc 23:110–119
Li YH, Shi GY, Wu HL (2006) The role of thrombomodulin in atherosclerosis: from bench to bedside. Cardiovasc Hematol Agents Med Chem 4:183–187
Conway EM (2012) Thrombomodulin and its role in inflammation. Semin Immunopathol 34:107–125
Shi CS, Shi GY, Hsiao SM et al (2008) Lectin-like domain of thrombomodulin binds to its specific ligand Lewis Y antigen and neutralizes lipopolysaccharide-induced inflammatory response. Blood 112:3661–3670
Kuo CH, Chen PK, Chang BI et al (2012) The recombinant lectin-like domain of thrombomodulin inhibits angiogenesis through interaction with Lewis Y antigen. Blood 119:1302–1313
Li YH, Chung HC, Luo CY et al (2010) Thrombomodulin is upregulated in cardiomyocytes during cardiac hypertrophy and prevents the progression of contractile dysfunction. J Card Fail 16:980–990
Tsai PY, Ka SM, Chang JM et al (2012) Antroquinonol differentially modulates T cell activity and reduces interleukin-18 production, but enhances Nrf2 activation, in murine accelerated severe lupus nephritis. Arthritis Rheum 64:232–242
Chao TK, Rifai A, Ka SM et al (2006) The endogenous immune response modulates the course of IgA-immune complex mediated nephropathy. Kidney Int 70:283–297
Ohse T, Pippin JW, Vaughan MR, Brinkkoetter PT, Krofft RD, Shankland SJ (2008) Establishment of conditionally immortalized mouse glomerular parietal epithelial cells in culture. J Am Soc Nephrol 19:1879–1890
Justo P, Sanz AB, Egido J, Ortiz A (2005) 3,4-Dideoxyglucosone-3-ene induces apoptosis in renal tubular epithelial cells. Diabetes 54:2424–2429
Susztak K, Raff AC, Schiffer M, Bottinger EP (2006) Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55:225–233
Heilbronn R, Weger S (2010) Viral vectors for gene transfer: current status of gene therapeutics. Handb Exp Pharmacol 197:143–170
Conway EM, Van de Wouwer M, Pollefeyt S et al (2002) The lectin-like domain of thrombomodulin confers protection from neutrophil-mediated tissue damage by suppressing adhesion molecule expression via nuclear factor kappaB and mitogen-activated protein kinase pathways. J Exp Med 196:565–577
Li YH, Kuo CH, Shi GY, Wu HL (2012) The role of thrombomodulin lectin-like domain in inflammation. J Biomed Sci 19:34
Van de Wouwer M, Plaisance S, De Vriese A et al (2006) The lectin-like domain of thrombomodulin interferes with complement activation and protects against arthritis. J Thromb Haemost 4:1813–1824
Wang H, Vinnikov I, Shahzad K et al (2012) The lectin-like domain of thrombomodulin ameliorates diabetic glomerulopathy via complement inhibition. Thromb Haemost 108:1141–1153
Donath MY, Shoelson SE (2011) Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 11:98–107
Vandanmagsar B, Youm YH, Ravussin A et al (2011) The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17:179–188
Masters SL, Dunne A, Subramanian SL et al (2010) Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol 11:897–904
Rustom R, Leggat H, Tomura HR, Hay CR, Bone JM (1998) Plasma thrombomodulin in renal disease: effects of renal function and proteinuria. Clin Nephrol 50:337–341
Aso Y, Fujiwara Y, Tayama K, Takebayashi K, Inukai T, Takemura Y (2000) Relationship between soluble thrombomodulin in plasma and coagulation or fibrinolysis in type 2 diabetes. Clin Chim Acta Int J Clin Chem 301:135–145
Uehara S, Gotoh K, Handa H (2001) Separation and characterization of the molecular species of thrombomodulin in the plasma of diabetic patients. Thromb Res 104:325–332
Kashihara N, Haruna Y, Kondeti VK, Kanwar YS (2010) Oxidative stress in diabetic nephropathy. Curr Med Chem 17:4256–4269
Na HK, Surh YJ (2008) Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc 46:1271–1278
Tsai PY, Ka SM, Chang JM et al (2011) Epigallocatechin-3-gallate prevents lupus nephritis development in mice via enhancing the Nrf2 antioxidant pathway and inhibiting NLRP3 inflammasome activation. Free Radic Biol Med 51:744–754
da Silva JL, Zand BA, Yang LM, Sabaawy HE, Lianos E, Abraham NG (2001) Heme oxygenase isoform-specific expression and distribution in the rat kidney. Kidney Int 59:1448–1457
Hung SY, Liou HC, Kang KH, Wu RM, Wen CC, Fu WM (2008) Overexpression of heme oxygenase-1 protects dopaminergic neurons against 1-methyl-4-phenylpyridinium-induced neurotoxicity. Mol Pharmacol 74:1564–1575
Ptilovanciv EO, Fernandes GS, Teixeira LC et al (2013) Heme oxygenase 1 improves glucoses metabolism and kidney histological alterations in diabetic rats. Diabetol Metab Syndr 5:3
Gilbert RE, Marsden PA (2008) Activated protein C and diabetic nephropathy. N Engl J Med 358:1628–1630
Funding
This study was supported by grants from the Department of Health, Executive Yuan (DOH97-TD-I-11-TM006), the Ministry of Economic Affairs (100-EC-17-A-19-S1-161) and the National Science Council (NSC 101-2321-B-016-003) of Taiwan, Republic of China.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
SMY acquired and analysed data, and drafted the manuscript. SMK performed the experiments using cultured podocytes, analysed the data and edited the manuscript. HLW analysed data and edited the manuscript. YCY engineered the AAV-THBDD1, analysed data and revised the manuscript. CHK generated the rTHBDD1, analysed data and revised the manuscript. KFH, GYS, YJH, FCH, SSY, YSS, SHL, CWW, YJH and CYY analysed the data and revised the manuscript. JSL designed, constructed and produced the AAV vectors, analysed data and revised the manuscript. AC designed the study and finalised the manuscript. All authors approved the final version of the manuscript. SMK and AC are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
THBDD1 expression after gene delivery. (a) THBDD1 mRNA levels in liver tissue obtained at 18 weeks when the mice were sacrificed. Black circles, WT (wild type, db/m). White circles, vector alone control db/db. Black triangle, AAV- THBDD1+db/db. (b) Serum THBDD1 levels detected in a time-course manner. † p < 0.005 versus vector alone control db/db. WT, wild type (db/m). (PDF 476 kb)
ESM Fig. 2
Renal Col-IV expression. (a) Col-IV expression by IHC. The arrow indicates the positive staining. Original magnification, ×400. Scoring of Col-IV expression in (b) the glomerulus or (c) tubulointerstitial compartment. *p < 0.05, and **p < 0.01. WT, wild type (db/m). NS, not significant. (PDF 767 kb)
ESM Fig. 3
Renal in situ ROS production. (a) DHE staining. The arrow indicates glomeruli. Original magnification, ×400. (b) Scoring of in situ ROS production as the percentage of positive cells.*p < 0.05, **p < 0.01, and † p < 0.005. WT, wild type (db/m). (PDF 208 kb)
ESM Fig. 4
Renal IL-1β expression. (a) IL-1β expression by IHC. The arrow indicates the positive staining. Original magnification, ×400. Scoring of IL-1β expression in the (b) glomerulus or (c) tubulointerstitial compartment. **p < 0.01, and † p < 0.005. WT, wild type (db/m). NS, not significant. (PDF 693 kb)
ESM Fig. 5
TUNEL for apoptosis (a) TUNEL staining of renal tissues at week 18 after injection. The arrow indicates positive staining. Original magnification, ×400. Scoring of positive cells in (b) glomerulus or (c) renal tubule.*p < 0.05, and **p < 0.01. gcs, glomerular cross section. WT, wild type (db/m). NS, not significant. (PDF 308 kb)
ESM Methods
(PDF 83.7 kb)
Rights and permissions
About this article
Cite this article
Yang, SM., Ka, SM., Wu, HL. et al. Thrombomodulin domain 1 ameliorates diabetic nephropathy in mice via anti-NF-κB/NLRP3 inflammasome-mediated inflammation, enhancement of NRF2 antioxidant activity and inhibition of apoptosis. Diabetologia 57, 424–434 (2014). https://doi.org/10.1007/s00125-013-3115-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-3115-6